
���� ��1����֤��ͼ�֮��ȷʵ�����˻�ѧ��Ӧ����Ҫѡ��һ�����һ�ּ��ָʾ����
��2��̼������ϡ���ᷴӦ�����ɶ�����̼���壻
��3������������̼���Ʒ�Ӧ������̼��Ƴ�����
��� �⣺��1����֤��ͼ�֮��ȷʵ�����˻�ѧ��Ӧ������ϡ����ͱ���ʯ��ˮ��Ӧ������ʵ��û�����Ե�������Ҫ����ָʾ������Ϊ���ձ������Թܣ��м�����������ʯ��ˮ�����뼸�η�̪��Һ���õι���ε���ϡ���ᣬ�����Ͻ��裨������Һ������Һǡ�ñ����ɫΪֹ��
��2��̼������ϡ���ᷴӦ�����ɶ�����̼���壬��ѧ����ʽΪ��2HCl+Na2CO3=2NaCl+H2O+CO2����
��3������������̼���Ʒ�Ӧ������̼��Ƴ�������ѧ����ʽΪ��Na2CO3+Ca��OH��2=CaCO3��+2NaOH��
�ʴ�Ϊ����1�����ձ������Թܣ��м�����������ʯ��ˮ�����뼸�η�̪��Һ���õι���ε���ϡ���ᣬ�����Ͻ��裨������Һ������Һǡ�ñ����ɫΪֹ��
��2��2HCl+Na2CO3=2NaCl+H2O+CO2����
��3��Na2CO3+Ca��OH��2=CaCO3��+2NaOH��
���� ���⿼������ͼ�ķ�Ӧ�ȣ���ɴ��⣬�����������е�֪ʶ���У�Ҫ��ͬѧ����ƽʱ��ѧϰ�м�ǿ����֪ʶ�Ĵ������Ա��ܹ����Ӧ�ã�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʱ�����������ù��Ǹ��� | |
| B�� | ���dz��õ�Ǧ�ʺ��У�ǦԪ��Ǧ�ʲ�Ҫ�����������ж� | |
| C�� | �������ij��������û���̿������ | |
| D�� | ��ijЩʳƷ��װ�пɳ��뵪���Է��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ����ϡ����������g�� | 10 | 10 | 10 | 10 |
| ʣ������������g�� | 9.10 | 8.45 | 7.80 | 7.80 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������ȼ�գ�4Fe+3O2$\frac{\underline{\;��ȼ\;}}{\;}$ 2Fe2O3 | |
| B�� | ������������ȼ�գ�CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$2H2O+CO2 | |
| C�� | ����ϡ���ᷴӦ��2Fe+6HCl�T2 Fe Cl 3+3H2�� | |
| D�� | ̿��ԭ����ͭ�ķ�Ӧ��C+2 CuO�TCO2��+2Cu |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
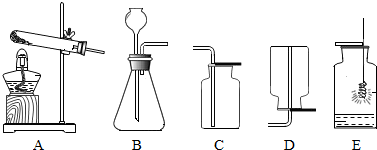
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ���ǵ��� | B�� | һ���Ǵ����� | C�� | һ���ǻ���� | D�� | һ�����ǻ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com