��2004?������þ����Ҫ�Ľ����ṹ���ϣ��������ڷɻ�����ҵ����ˮ��þԪ�ص�����������������Ԫ�غ���Ԫ�أ��ӵ���λ��Ŀǰ�����ϴ�þ���ǴӺ�ˮ����ȡ�ģ�ijУ��ѧ�С���ͬѧ�����ⶨ��ˮ���Ȼ�þ�ĺ������ס��ҡ�����λͬѧ�ֱ����ʵ�飬ʵ�����������±�������ֻ��һλͬѧ�õĺ�ˮ��Ʒ�������������������Һǡ����ȫ��Ӧ���۲�����������ݣ��ش��������⣺
| | �� | �� | �� |
| ��ȡ��ˮ��Ʒ��������g�� | 100 | 100 | 150 |
| ����NaOH��Һ��������g�� | 20 | 10 | 10 |
| ��Ӧ�����ij������������g�� | 0.29 | 0.29 | 0.29 |
��1������Һǡ����ȫ��Ӧ����______��ʵ�飮
����ס����ҡ�������
��2�����㺣ˮ��Ʒ��MgCl
2����������������������ȷ��0.001��
��3����Ҫ�Ӻ�ˮ����ȡ2.4tþ�������躣ˮ���ٶ֣�

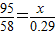
 =0.475g��
=0.475g��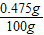 ×100%=0.475%��
×100%=0.475%��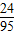 ×100%=2.4t��
×100%=2.4t��

