
£Ø8·Ö£©ĪŅŹŠÄ³»Æ¹¤³§ÅŷŵķĻŅŗÖŠŗ¬ÓŠĮņĖįĶŗĶĮņĖįŃĒĢś£®Ä³»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§Č”ĮĖŹŹĮæµÄÉĻŹö·ĻŅŗѳʷ£¬½«Ņ»¶ØĮæŠæ·Ū¼ÓČėѳʷ֊£¬³ä·Ö·“Ó¦ŗó¹żĀĖ£¬µĆµ½ĀĖŅŗAŗĶ¹ĢĢåB£®Ēė»Ų“šĻĀĮŠÓŠ¹ŲĪŹĢā£ŗ
£Ø1£©¹ŲÓŚĀĖŅŗAĖłŗ¬ČÜÖŹµÄ²ĀĻėÖŠ£¬²»ŗĻĄķµÄŹĒ £ØĢīŠ“ŠņŗÅ£©£®
¢Ł²ĀĻėŅ»£ŗÖ»ÓŠĮņĖįŠæ£» ¢Ś²ĀĻė¶ž£ŗĮņĖįŠæ”¢ĮņĖįŃĒĢś£»
¢Ū²ĀĻėČż£ŗĮņĖįŠæ”¢ĮņĖįŃĒĢś”¢ĮņĖįĶ£» ¢Ü²ĀĻėĖÄ£ŗĮņĖįŠæ”¢ĮņĖįĶ
£Ø2£©Éč¼Ę¼ņµ„ŹµŃé£¬Č·¶Ø”°²ĀĻė¶ž”±¶ŌÓ¦µÄ¹ĢĢåBµÄ³É·Ö£ŗ
ŹµŃé²Ł×÷£ŗ
ŹµŃéĻÖĻóŗĶ½įĀŪ£ŗ
£Ø3£©Š“³öĖłÉę¼°µ½µÄ»Æѧ·“Ó¦·½³ĢŹ½
£Ø4£©·“Ó¦ŗóĪö³ö½šŹōµÄÖŹĮæ £ØŃ”Ģī”°Ņ»¶Ø”±»ņ”°²»Ņ»¶Ø”±£©±Č²Ī¼Ó·“Ó¦µÄ½šŹōÖŹĮ抔£®
£Ø1£©¢Ü£» £Ø2£©ÓĆ“ÅĢśĪüŅż£¬ÓŠŗŚÉ«·ŪÄ©±»ĪüŅż£¬ĖµĆ÷ĮĖBŹĒĶÓėÉŁĮæĢśµÄ»ģŗĻĪļ£¬Čōƻӊ±»ĪüŅż£¬ĖµĆ÷ĮĖBŹĒĶ£» £Ø3£©Zn+CuSO4==ZnSO4+Cu £»Zn+FeSO4==ZnSO4+Fe£» £Ø4£©Ņ»¶Ø£®
½āĪöŹŌĢā·ÖĪö£ŗ½šŹō»ī¶ÆŠŌÓÉĒæµ½ČõµÄĖ³Šņ£ŗŠæ£¾Ģś£¾H£¾Ķ£¬ĻņĮņĖįŃĒĢś”¢ĮņĖįĶµÄ»ģŗĻČÜŅŗÖŠ¼ÓČėŅ»¶ØĮæµÄŠæ·Ū£¬ĶŹ×Ļȱ»ÖĆ»»³öĄ“£¬“żĶ±»ĶźČ«ÖĆ»»ŗó£¬ĢśæŖŹ¼±»ÖĆ»»£»
£Ø1£©¢Łµ±ŠæŹĒ×ćĮæµÄ£¬ÄÜ°ŃČÜŅŗÖŠµÄĶ”¢ĢśČ«²æÖĆ»»³öĄ“£¬ĀĖŅŗÖŠÖ»ÓŠĮņĖįŠæ£¬¹Ź²ĀĻėŅ»ŗĻĄķ£»¢Śµ±ŠæŹĒ²»×ćĮæµÄ£¬ČÜŅŗÖŠµÄĶČ«²æÖĆ»»³öĄ“£¬·ĻŅŗÖŠµÄĢśĆ»ÓŠ±»ÖĆ»»»ņ²æ·Ö±»ÖĆ»»£¬ŌņĀĖŅŗÖŠÓŠĮņĖįŠæ”¢ĮņĖįŃĒĢś£¬¹Ź²ĀĻė¶žŗĻĄķ£»¢Ūµ±ŠæŹĒ²»×ćĮæµÄ£¬²»ÄÜ°ŃČÜŅŗÖŠµÄĶČ«²æÖĆ»»³öĄ“£¬ĀĖŅŗÖŠÓŠĮņĖįŠæ”¢ĮņĖįŃĒĢś”¢ĮņĖįĶ£¬¹Ź²ĀĻėČżŗĻĄķ£»¢ÜÓÉÓŚĢśµÄ»ī¶ÆŠŌ“óÓŚĶ£¬ĶŹ×Ļȱ»ÖĆ»»³öĄ“£¬²»æÉÄÜČÜŅŗÖŠÓŠĮņĖįĶ£¬Ć»ÓŠĮņĖįŃĒĢś£¬¹Ź²ĀĻėĖIJ»ŗĻĄķ£»
£Ø2£©ÓÉĢāŅāæÉÖŖ£¬µ±ĀĖŅŗAÖŠŗ¬ÓŠĮņĖįŠæ”¢ĮņĖįŃĒĢśŹ±£¬¹ĢĢåBæÉÄÜŹĒĶ»ņĶÓėÉŁĮæĢśµÄ»ģŗĻĪļ£»ÓÉÓŚĢśÄܱ»“ÅĢśĪüŅż£¬ĖłŅŌÉč¼ĘŹµŃéŹĒ£ŗÓĆ“ÅĢśĪüŅż£¬ÓŠŗŚÉ«·ŪÄ©±»ĪüŅż£¬ĖµĆ÷ĮĖBŹĒĶÓėÉŁĮæĢśµÄ»ģŗĻĪļ£¬Čōƻӊ±»ĪüŅż£¬ĖµĆ÷ĮĖBŹĒĶ£»£Ø3£©Ņ»¶Ø·¢ÉśµÄ·“Ó¦ŹĒŠæÓėĮņĖįĶÉś³ÉĮņĖįŠæŗĶĶ£¬Zn+CuSO4==ZnSO4+Cu £»Šæ×ćĮæŹ±£¬Ņ²·¢ÉśŠæÓėĮņĖįŃĒĢśµÄ·“Ó¦£¬Éś³ÉĮņĖįŠæŗĶĢś£ŗZn+FeSO4==ZnSO4+Fe£»
£Ø4£©ÓÉÓŚŠæ²»ĀŪÓėĮņĖįĶ»¹ŹĒÓėĮņĖįŃĒĢś·“Ó¦£¬ÓÉ·“Ó¦µÄ·½³ĢŹ½ø÷ĪļÖŹµÄÖŹĮæ±ČæÉÖŖ£¬²Ī¼Ó·“Ó¦µÄ½šŹōŠæ±ČÉś³ÉµÄ½šŹōĶ»ņĢśµÄÖŹĮæ“ó£¬ĖłŅŌ·“Ó¦ŗóĪö³ö½šŹōµÄÖŹĮæŅ»¶Ø±Č²Ī¼Ó·“Ó¦µÄ½šŹōÖŹĮ抔£®
æ¼µć£ŗ½šŹō»ī¶ÆŠŌĖ³Šņ¼°ĘäÓ¦ÓĆ


 Ņ»¾ķøć¶ØĻµĮŠ“š°ø
Ņ»¾ķøć¶ØĻµĮŠ“š°ø ĆūŠ£×÷Ņµ±¾ĻµĮŠ“š°ø
ĆūŠ£×÷Ņµ±¾ĻµĮŠ“š°ø ĒįĒɶį¹ŚÖܲāŌĀæ¼Ö±ĶØĆūŠ£ĻµĮŠ“š°ø
ĒįĒɶį¹ŚÖܲāŌĀæ¼Ö±ĶØĆūŠ£ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÕšŌÖ¹żŗó£¬ŅūÓĆĖ®µÄĻū¶¾É±¾ś³ÉĪŖŅÖÖĘ“ó¹ęÄ£“«Č¾ŠŌ¼²²”±¬·¢µÄÓŠŠ§·½·ØÖ®Ņ»”£ĘÆ°×·ŪŹĒ³£ÓƵÄĻū¶¾¼Į”£ĘÆ°×·ŪµÄÖ÷ŅŖ³É·ÖŹĒ“ĪĀČĖįøĘ[Ca(ClO)2]ŗĶĀČ»ÆøĘ£¬ÓŠŠ§³É·ÖŹĒ“ĪĀČĖįøĘ”£ĘÆ°×·ŪµÄĘÆ°×ŌĄķŹĒ“ĪĀČĖįøĘÓėĖį·“Ó¦²śÉśÓŠĘư׊ŌµÄĪļÖŹ“ĪĀČĖį£ŗ””””
£Ø1£©¹¤ŅµÉĻ½«ĀČĘųĶØČėŹÆ»ŅČé[Ca(OH)2]ÖĘČ”ĘÆ°×·Ū£¬Ķ¬Ź±ÓŠĖ®Éś³É£¬Ęä·“Ó¦»Æѧ·½³ĢŹ½ĪŖ£ŗ”” ”£
£Ø2£©ĘÆ°×·ŪČÜÓŚĖ®ŗó£¬ŹÜæÕĘųÖŠµÄCO2×÷ÓĆ£¬¼“²śÉśÓŠĘÆ°×”¢É±¾ś×÷ÓƵēĪĀČĖį£¬»Æѧ·½³ĢŹ½ĪŖ£ŗ”” ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
»Æѧ¾ĶŌŚĪŅĆĒÉķ±ß£¬ĖüÓėĪŅĆĒµÄÉś»īĻ¢Ļ¢Ļą¹Ų”£ĻÖÓŠ¢ŁŹģŹÆ»Ņ”¢¢Ś“æ¼ī”¢¢ŪÉÕ¼ī”¢¢ÜĻõĖį¼Ų”¢¢ŻŃĪĖį”¢¢ŽĀĮ”¢¢ßĢśµČĪļÖŹ£¬ĒėÓĆĻąÓ¦ĪļÖŹµÄ»ÆѧŹ½ĢīæÕ”£
£Ø1£©ČĖĢåĪøŅŗÖŠŗ¬ÓŠµÄĖįŹĒ £» £Ø2£©Ņ×ĪüĖ®¶ų³±½āµÄ¼īŹĒ £»
£Ø3£©ÉĻŹöĪļÖŹÖŠŹōÓŚÅ©ĢļŹ©ÓƵÄø“ŗĻ·ŹµÄĪļÖŹŹĒ £»
£Ø4£©ÄÜÓŚøÄĮ¼ĖįŠŌĶĮČĄµÄŹĒ £ŗ
£Ø5£©µŲæĒÖŠŗ¬ĮæĪ»¾ÓµŚ¶žµÄ½šŹōŌŖĖŲŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø6·Ö£©¢Å “ÓÄÜŌ“ŹĒ·ńæÉŅŌŃ»·ŌŁÉśµÄ½Ē¶Čæ“£¬æÉŅŌ½«ÄÜŌ“·ÖĪŖæÉŌŁÉśÄÜŌ“ŗĶ²»æÉŌŁÉśÄÜŌ“£¬Ę©Čē ¾ĶŹĒ²»æÉŌŁÉśÄÜŌ“”£ČÕŅęŃĻ¾žµÄÄÜŌ“Ī£»ś“Ł½ųĮĖæÉŌŁÉśÄÜŌ“µÄŃŠ¾æ”£ÓŠ×ؼŅĢį³ö£ŗČē¹ūÄܹ»ĄūÓĆĢ«ŃōÄÜŹ¹Č¼ĮĻČ¼ÉÕ²śĪļ£¬ČēCO2”¢H2O”¢N2µČÖŲŠĀ×éŗĻ£ØČēĶ¼£©£¬æÉŅŌ½ŚŌ¼Č¼ĮĻ£¬»ŗ½āÄÜŌ“Ī£»ś”£ŌŚ“Ė¹¹ĻėµÄĪļÖŹŃ»·ÖŠĢ«ŃōÄÜ×īÖÕ×Ŗ»ÆĪŖ ÄÜ”£
¢Ę ŹŌøł¾ŻĶ¼ĖłŹ¾Š“³ö°±Ęų×÷ĪŖČ¼ĮĻČ¼ÉյĻÆѧ·½³ĢŹ½£ŗ
ӣ
¢Ē gCH3OH£Ø¼×“¼£©ÖŠĢ¼ŌŖĖŲÖŹĮæÓė16g¼×ĶéÖŠĢ¼ŌŖĖŲÖŹĮæĻąµČ”£
¢Č ¶ą¾§¹čŹĒµ„ÖŹ¹čµÄŅ»ÖÖŠĪĢ¬”£ŌŚĢ«ŃōÄÜĄūÓĆÉĻ£¬¶ą¾§¹č·¢»ÓמŽ“óµÄ×÷ÓĆ”£
¶ą¾§¹čŹōÓŚ ”£
A£®ø“ŗĻ²ÄĮĻ B.ĪŽ»ś²ÄĮĻ C.ŗĻ³É²ÄĮĻ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø4·Ö£©ĻÖÓŠH”¢O”¢Na”¢S”¢CĪåÖÖŌŖĖŲ£¬Ēė“ÓÖŠŃ”ŌńŗĻŹŹµÄŌŖĖŲ£¬°“ŅŖĒóŹéŠ“»ÆѧŹ½”£
£Ø1£©Ņ»ÖÖ½šŹōŃõ»ÆĪļ £»£Ø2£©Ņ»ÖÖŃĪ £»
£Ø3£©Ņ»ÖÖ¼ī £»£Ø4£©Ņ»ÖÖĘųĢåµ„ÖŹ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø5·Ö£©Š“³öĻĀĮŠ·“Ó¦·½³ĢŹ½£¬²¢ŌŚĄØŗÅÄŚ×¢Ć÷»ł±¾·“Ó¦ĄąŠĶ”£
¢Å Į×µÄČ¼ÉÕ __________________________________________ ( )
¢Ę ÓĆĻ”ŃĪĖį³żĢśŠā____________________________________ ( )
£Ø3£©¾²éŌÄ׏ĮĻµĆÖŖ£¬ŅŅČ²£ØC2H2£©ĘųĢåæÉÓĆĖ®ŗĶCaC2¹ĢĢå·“Ó¦ÖĘČ””£Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø4·Ö£©øł¾ŻŅŖĒ󊓳öĻĀĮŠ»Æѧ·½³ĢŹ½£¬²¢×¢Ć÷»ł±¾·“Ó¦ĄąŠĶ”£
¢ÅĢśŗĶĮņĖįĶČÜŅŗ·“Ó¦___________________________________£Ø £©·“Ó¦
¢ĘĒāŃõ»ÆÄĘŗĶĻ”ŃĪĖį·“Ó¦_________________________________£Ø £©·“Ó¦
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø5·Ö£©·ŪĆŗ»ŅŹĒŅ»ÖÖ»šÉ½»ŅÖŹ²ÄĮĻ£¬Ą“Ō“ÓŚĆŗÖŠµÄĪŽ»ś×é³É£¬“Ó·ŪĆŗ»ŅÖŠÄܹ»»ńµĆAl2O3£¬ŅŃÖŖij»šĮ¦·¢µē³§µÄ·ŪĆŗ»ŅÖŠµÄ»Æѧ³É·ÖČēĻĀ±ķ£ŗ
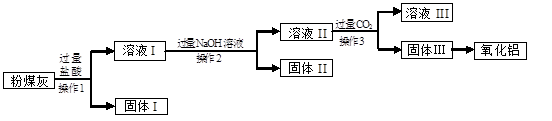
ÉĻŹöĮ÷³ĢÖŠÉę¼°µÄ²æ·Ö·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
Al(OH)3 + NaOH ="==" NaAlO2 + 2H2O £ØNaAlO2æÉČÜÓŚĖ®£©”£
Ēė»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©ÉĻŹöŃõ»ÆĪļÖŠÄÜÓėĖ®·“Ó¦µÄŹĒ ”£
£Ø2£©·ŪĆŗ»ŅÓė¹żĮæµÄŃĪĖį·“Ó¦µÄÖ÷ŅŖ»Æѧ·½³ĢŹ½ŹĒ £ØŠ“³öĘäÖŠµÄŅ»øö£©”£
£Ø3£©²Ł×÷2µÄĆū³ĘŹĒ ”£
£Ø4£©¹ĢĢå¢ņŗ¬ÓŠµÄÖ÷ŅŖĪļÖŹµÄ»ÆѧŹ½ĪŖ ”£
£Ø5£©ČÜŅŗ¢ņÓė¹żĮæµÄCO2·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
NaAlO2 + CO2 + 2H2O ="==" Al£ØOH£©3”ż + ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓĆŹż×ÖŗĶ»Æѧ·ūŗÅĢīæÕ£ŗ
¢Ł3øöĖ®·Ö×Ó £»
¢Ś2øöĮņŌ×Ó £»
¢ŪÕż3¼ŪµÄĀĮŌŖĖŲ £»
¢ÜøĘĄė×Ó ”£
¢Ż³£ĪĀĻĀ³ŹŅŗĢåµÄ½šŹōµ„ÖŹ £»
¢ŽĢģČ»“ęŌŚµÄ×īÓ²ĪļÖŹ £»
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com