
ijУ��ѧ��ȤС��ѧϰ���������ȡ���ռ������֪ʶ�����ܽᣬ�����������Ŀ��
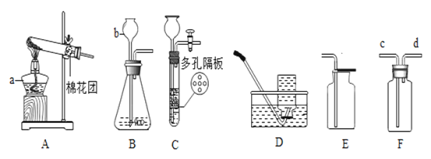
(1)д�������������ƣ�b________________��
(2)ʵ��������Aװ����ȡ��������Ӧ�Ļ�ѧ����ʽΪ_______________________�����ռ�������������֤����ȼ�ղ����ʵ�飬���ѡ���ռ�װ��Ϊ_______(��A��E��ѡ)��
(3)ʵ������ȡ����ʱ����Cװ�ô���Bװ���ڲ���������ŵ���_______________������װ��F��װ��ˮ���ռ���������Ӧ��______��ͨ��(��c��d)��ʵ������ȡ�����Ļ�ѧ����ʽ��_______________________��
(4)��Ҫ����CO2��CO�Ļ�����壬�ɽ�����ͨ����ͼװ��(Һ��ҩƷ������)�������������£�
�ٹرջ���______������_____(��a��b)��ͨ�������壬���ռ���__________���塣
��Ȼ��______________________(�����)���ֿ��ռ�����һ�����塣
����֪��ʵ���ҿ����ü����Ȼ�狀��������ƵĻ������ȡ�������������ܶȱȿ�����С���ж�����������ˮ������ˮ���γɰ�ˮ��ijͬѧ����ͼFװ�����ռ������������________�˽�(ѡ��c����d��)��Ϊ��ֹ������Ⱦ����һ�˽���ͼ1��ʾ��װ��(ʢ�ŵ�Һ���Ϊˮ)�����п��������ն��ఱ����װ����__________________��

��ͼ2װ�ÿ������������ɵ�O2������������װ�������Եķ����ǣ�������Ͳ��Ļ�������ʱ��������________________________________��˵�����������á�
 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ������2017����꼶��һ��������⻯ѧ�Ծ� ���ͣ���ѡ��
����������ѧϰ��ѧ���õ�һ�ַ���������������ȷ����
A. ˮ�����������Ԫ����ͬ�����Ի�ѧ����Ҳ��ͬ
B. ������������������˵�����������������κ����ʷ�Ӧ
C. ij����ȼ������CO2��H2O��������ʵ������һ������̼����Ԫ��
D. �û���Ӧ���е������ɣ������е������ɵĻ�ѧ��Ӧһ�����û���Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2019����꼶��ѧ�����п��Ի�ѧ�Ծ� ���ͣ���ѧ̽����
��ѧ��ȤС������ʦָ���£�Χ������кͷ�Ӧ��չ̽�����
����ʾʵ�飩��һ����ϡ�����������������Һ�У������Ͻ��裬û�й۲쵽������������ȷʵ���������µĻ�ѧ��Ӧ��H2SO4+2NaOH=Na2SO4+2H2O��
��������⣩��Ӧ����Һ�����ʵijɷ���ʲô��
����������裩��ͬѧ�IJ������£�
����I��ֻ��Na2 SO4�� �������____��
�������Na2SO4��NaOH �� ���������Na2SO4��H2SO4��NaOH
��ͬѧ��Ϊ���������������������____��
���������ϣ�BaCl2+Na2SO4=2NaCl+BaSO4�� ��BaCl2+H2SO4=2HCl+BaSO4����
��ʵ����֤��Ϊ����֤����IJ��룬��ͬѧ�������������ʵ�鷽����
��ȡ������ʯ����Һ | ��ȡ������CuSO4��Һ | ��ȡ������BaCl2��Һ | |
���� | ____ | ____ | ������ɫ���� |
���� | ��Һ�к���NaOH | ��Һ�к���NaOH | ��Һ�к���H2SO4 |
���ó����ۣ�ͬѧ�Ǿ������ۺ���Ϊ������������
�����۷�˼��
��1��д�����������йط�Ӧ�Ļ�ѧ����ʽ��____��
��2�������۲���֤����Һ�к���H2SO4��ԭ����____��
��3��Ҫ֤����Ӧ�����Һ���Ƿ���H2SO4������ѡ��____������ĸ����
A ��̪��Һ B pH��ֽ C CuO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ʡ������2018-2019ѧ����꼶��ѧ������֪ʶ��⻯ѧ�Ծ� ���ͣ�������
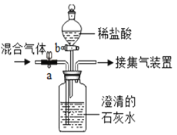
Ϊ�˲ⶨijʯ��ʯ��̼��Ƶ�����������ȷ��ȡ12.5gʯ��ʯ��Ʒ�����ձ��У������м�������ϡ���ᣨ�����в�����Ԫ�أ��������ᷴӦ��Ҳ������ˮ����ʵ���õ�������ͼ��ʾ��
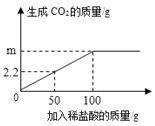
��1������Ʒ��ȫ��Ӧ�����ɶ�����̼������Ϊ_____��
��2�����ʯ��ʯ��Ʒ��̼��Ƶ���������_____��
��3��������50g����ʱ���ձ��������и�Ԫ������Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ʡ������2018-2019ѧ����꼶��ѧ������֪ʶ��⻯ѧ�Ծ� ���ͣ�ʵ����
������ͼ�ش�����
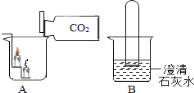
��1����������̼�����ձ��У�����______�� �����������ԭ���ǣ���______����______��
��2����һ������������̼���Թܵ�����ʢ�г���ʯ��ˮ���ձ��У����Թ۲쵽��������________________��________________��
��3�������ж�����̼�ĺ������࣬���������ЧӦ������ȫ���ů�����������Ӷ�����̼�ĺ���������������룮Ϊ�˻�������е�CO2���������ӣ����½�����е���______������ĸ����
A ����̫���ܡ�ˮ�ܡ����ܡ������ܵ�������Դ
B ��ֹʹ��ú��ʯ�͡���Ȼ���ȿ���ȼ��
C �ᳫֲ�����֣���ֹ�ҿ��ķ�
D �����ؿ���˫��ʹ��ֽ�ţ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ʡ������2018-2019ѧ����꼶��ѧ������֪ʶ��⻯ѧ�Ծ� ���ͣ���ѡ��
�����й�̼��̼�Ļ�����˵��������ǣ�������
A. ���ʯ��ʯī��C60����̼Ԫ����ɵĵ���
B. �ڼ��Ȼ���µ������£�CO����������������ﷴӦ
C. ������̼��ʹ��ɫʯ����Һ��죬˵��������̼��������
D. ˮī���ɳ��ñ��治��ɫ����Ϊ�ڳ�����̼�Ļ�ѧ���ʲ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ʡ������2018-2019ѧ����꼶��ѧ������֪ʶ��⻯ѧ�Ծ� ���ͣ���ѡ��
��ͼ��ʾ��ʵ��������о�ȼ�յ�����������˵������ȷ���ǣ� ��
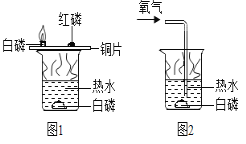
A. ͼ1��ͼ2��ˮ�°��Ա�˵��ȼ�ձ���Ҫ����������
B. ͼ1��ͭƬ�ϵİ��ͺ��Ա�˵��ȼ�ձ���Ҫ����������
C. ͼ1��ͭƬ�ϵĺ���ˮ�µİ��Ա�˵��ȼ�ձ���ﵽ��ȼ����Ż��
D. ����ʵ���ձ��е���ˮֻ�������¶ȵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ��̨��2018����꼶��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��ѧ�������������ϢϢ��أ���ش���������
��1�������м�����ë�����ߵķ�����_______��
��2������_______�������ƣ���������ף���۵�ʳ�����Ƿ��е��ۡ�
��3����Ԫ������������һ��_______�����������������Ԫ�أ������˳���ţ�̿���Ԥ��_______��
��4����ϴ�ྫ�����ɳ�ȥ����ϵ����ۣ�����������ϴ�ྫ��_______���ã�ˮ�dz��õ��ܼ���ij�ƾ���C2H5OH����ˮ��Һ���������ܼ�����ԭ�Ӹ�����Ϊ1��2�������Һ�����ʵ���������Ϊ_______����ȷ��1%��
��5�����ʵ�ʹ�ã�ʹ����ʵ�����ɴ�ͳũҵ���ִ�ũҵ�Ŀ�Խ��������李�����ء����ء�����ء��������صȻ����У����ڸ��Ϸʵ���_______�֣�ij����炙��ʰ�װ����ע��������Ϊ30%����÷��ϵĴ�����_______����ȷ��1%������ʵ�����м����̬���ʵķ�����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2019����꼶��ѧ�ڵڶ���ģ�⿼�Ի�ѧ�Ծ� ���ͣ�������
�ںϳɰ���ҵ�У�����̼�����Һ���ղ����Ķ�����̼�õ�̼����أ���Ӧ�Ļ�ѧ����ʽΪ��K2CO3+CO2+H2O=2KHCO3������̼�����Һ��ͨ�������̼��ǡ����ȫ��Ӧ���õ�������������Ϊ10%����Һ50 g���Լ��㣺
��1�����Ѹ�50 g��Һϡ�ͳ�������������Ϊ4%����Һ�����ˮ��������___g��
��2��ԭ̼�����Һ�����ʵ����������������ȷ��0.1%����________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com