
| ���ʵ��������� | ��Һ���ܶ� | |
| ��һ����Һ | ��1 | ��1 |
| �ڶ�����Һ | ��2 | ��2 |
 ���м��㣮
���м��㣮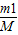
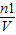 =
=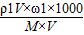 =
=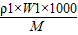
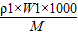
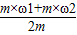 ×100%=��
×100%=��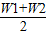 ��×100%��
��×100%��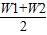 ��×100%
��×100%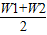 ��×100%
��×100%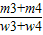 ×100%=
×100%=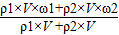
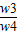 =
= +
+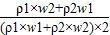 ����1����2�� ������1����2�����4����3��
����1����2�� ������1����2�����4����3��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ���� | ��ʵ�鷽�� | ���� | CO��CuO��Ӧ�Ļ�ѧ����ʽ |
| ��ĩΪCu | ����Ӧ | CuO+CO=Cu+CO2 | |
| ��ĩ�ܽ⣬��Һ�����ɫ�����ɺ�ɫ��ĩ | 2CuO+CO=Cu2O+CO2 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���ʵ��������� | ��Һ���ܶȣ�g?cm-3�� | |
| ��һ����Һ | ��1 | ��1 |
| �ڶ�����Һ | ��2 | ��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2012?��Ӫ����Ӫ�мƻ���5��ʱ�䣬���ֽ���30����̬�ֳ���ʵ�ֳ���ɭ�ֻ������غ���ˮ������Ŀ�꣮Ϊ��֤��ֲ��ľ�ijɻ��ʣ����ϻ����˵�ƿ������ͼ����ij����С����Ե�ƿ����Һ��ɷֽ���̽�����쿴��ƿ����˵���ܼ�û�о���ijɷ֣��������жϿ϶���һ��ֲ��Ӫ��Һ����������������������ϣ�
��2012?��Ӫ����Ӫ�мƻ���5��ʱ�䣬���ֽ���30����̬�ֳ���ʵ�ֳ���ɭ�ֻ������غ���ˮ������Ŀ�꣮Ϊ��֤��ֲ��ľ�ijɻ��ʣ����ϻ����˵�ƿ������ͼ����ij����С����Ե�ƿ����Һ��ɷֽ���̽�����쿴��ƿ����˵���ܼ�û�о���ijɷ֣��������жϿ϶���һ��ֲ��Ӫ��Һ����������������������ϣ�| �¶�/�� | 0 | 20 | 50 |
| ����� | 13.3 | 31.6 | 85.5 |
| ����� | 23.6 | 38.8 | 50.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ѧѧϰһ��ͨ����ѧ�����꼶�²� �˽̿α� ���ͣ�038
������60 g 10%��NaOH��Һ�������зֱ�ͨ���������ȵ�CO2������ҺA��B��Ȼ����A��B��Һ�зֱ������������Ũ�ȵ�ϡ���ᣬ�ų�CO2�������ͼ��ʾ����A��B��Һ�зֱ�ʲô���ʣ��������ֱ��Ƕ���?
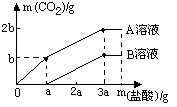
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com