��2013?��ģ�⣩��ȤС��ι�ij�Ƽ���������Ϣ���������������о���
���������ϡ�
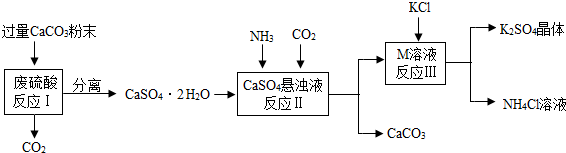
�ٴ����к����������������ʣ�MgCl
2��CaCl
2�������������ʣ�
�ڷ�Ӧԭ����NaCl�����ͣ�+NH
3+CO
2+H
2O=NaHCO
3��+NH
4Cl����������ľ���A��ּ��ȣ����Ƶô��
��NH
4Cl
NH
3��+HCl����
����ˮ����ͭ��ˮ����
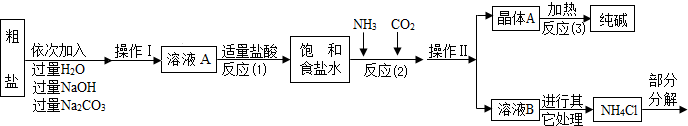
�ݲ���������������ͼ��ʾ��
���������ۡ�
��1����д����������������Һ��������Ӧ�Ļ�ѧ����ʽ
2NaOH+MgCl2�TMg��OH��2��+2NaCl��
2NaOH+MgCl2�TMg��OH��2��+2NaCl��
��
�ڲ����������Ϊ
����
����
��
�۷�Ӧ��1���м����������������
��ȥ�������������ƺ�̼����
��ȥ�������������ƺ�̼����
��
�ܷ�Ӧ��2����Ϊ��߲��ʣ����������˳����
B
B
������ĸ����
A����ͨ�������̼��ͨ���� B����ͨ�백����ͨ������̼
��2���������������в���ѭ��ʹ�õ���
D
D
������ĸ����
A��CO
2 B��NH
3 C��HCl D��NaOH
�����̽��һ��
��3���پ���A���ȷֽ�Ļ�ѧ����ʽΪ
2NaHCO
3Na
2CO
3+H
2O+CO
2����
2NaHCO
3Na
2CO
3+H
2O+CO
2����
��
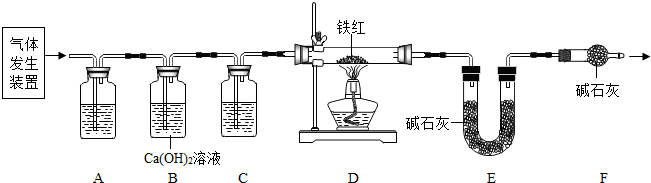
�����ʵ����鴿����Ʒ���Ƿ���о���A������±���

| ѡ���װ�� |
ʵ������ |
ʵ����� |
AB AB |
B�г���ʯ��ˮ������� B�г���ʯ��ˮ������� |
��Ʒ��������A |
�����̽������
��4��ȡ������Ʒ��ˮ�ܽ⣬�����м������ϡHNO
3���ٵμ�AgNO
3��Һ���а�ɫ���������������ķ���ʽΪ
AgNO3+NaCl�TAgCl��+NaNO3
AgNO3+NaCl�TAgCl��+NaNO3
��ȷ��������Ʒ��������NaCl��
�����̽������
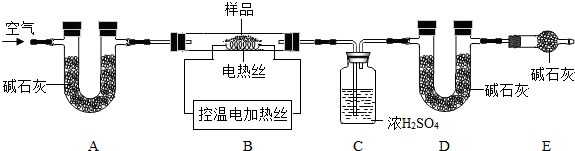
��5��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飺
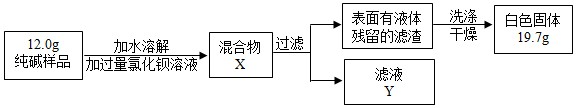
���жϼ���BaCl
2��Һ�Ƿ�����ĺ��ʷ�����
A
A
���۲������жϣ�
A�����û����X�����ϲ���Һ���ٵ�BaCl
2��Һ
B������ҺY�еμ�BaCl
2��Һ
���ж������Ƿ�ϴ�Ӹɾ����ɲ�ȡ��ϴ��Һ�еμ�
BC
BC
���۲������жϣ�
A��BaCl
2��Һ B��ϡH
2SO
4 C��Na
2CO
3��Һ D��ϡHCl
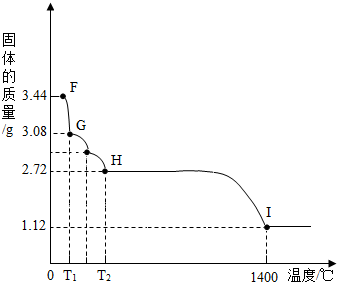
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ
88.3%
88.3%
��д��������̣�



 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�