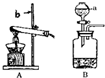
ʵ���ҳ��ø�����ط�ĩ��ȡ������װ��A����Ҳ�����ù���������Һ�ʹ���������������ȡ������װ��B����
��1��д��ʵ������˫��ˮ�Ͷ������̻����ȡ�����Ļ�ѧ����ʽ��
��2��װ��A��Ӧ���Թܿڷ�
һ�����ɵ���
һ�����ɵ���
���Է�������ط�ĩ���뵼�ܣ�
��3�����װ��B�еķ�Ӧ�ܾ��ң�Ӧ�ò�ȡ�İ�ȫ��ʩ��
��ʱ�رշ�Һ©���Ļ���������μ��ٶ�
��ʱ�رշ�Һ©���Ļ���������μ��ٶ�
��
��4����Aװ���Ʊ�����Ӧ���ǵ�������
��Ӧ���״̬�ͷ�Ӧ���������Ƿ��ǹ̹̼����ͣ�
��Ӧ���״̬�ͷ�Ӧ���������Ƿ��ǹ̹̼����ͣ�
��
��5��С��ͬѧ��
B
B
װ�ã��A����B����������ȡ������̼��д��ʵ������ȡ������̼�Ļ�ѧ����ʽ
CaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
��
��6����������Ĺ�������������������Һ���������ŷų��ķ�Һ������ȥ������������Ϊ15%������������Һ40g�����кͷ����������������������Ϊ����g��
�⣺���кͷ������������������Ϊx
H
2SO
4+2NaOH�TNa
2SO
4+2H
2O
9880
x40g��15%
=
��ã�x=7.35g
���кͷ������������������Ϊ7.35g
�⣺���кͷ������������������Ϊx
H
2SO
4+2NaOH�TNa
2SO
4+2H
2O
9880
x40g��15%
=
��ã�x=7.35g
���кͷ������������������Ϊ7.35g
��

 ʵ���ҳ��ø�����ط�ĩ��ȡ������װ��A����Ҳ�����ù���������Һ�ʹ���������������ȡ������װ��B����
ʵ���ҳ��ø�����ط�ĩ��ȡ������װ��A����Ҳ�����ù���������Һ�ʹ���������������ȡ������װ��B����

