
| ʵ����� | a | b | c |
| �Ͻ�����/mg | 510 | 765 | 918 |
| �������/mL | 560 | 672 | 672 |
���� ��1�����ݷ����Ļ�ѧ��Ӧ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����NaAlO2+CO2+2H2O=NaHCO3+Al��OH��3����2Al��OH��3$\frac{\underline{\;\;��\;\;}}{\;}$Al2O3+3H2O�����õ��Ĺ��������������������䣬˵������������Ԫ�ص�������������þԪ�ص�����������
��2�����ݱ����ṩ�������ж����ᷴӦ�������ٽ��м��㣮
��� �⣺��1��2Al+2NaOH+2H2O�T2NaAlO2+3H2����NaAlO2+CO2+2H2O=NaHCO3+Al��OH��3����2Al��OH��3$\frac{\underline{\;\;��\;\;}}{\;}$Al2O3+3H2O�����õ��Ĺ��������������������䣬˵������������Ԫ�ص�������������þԪ�ص���������������Ԫ�ص�����������������������Ԫ�ص�����������$\frac{27��2}{27��2+16��3}��100%$��52.9%��
��2���ݱ�����Կ�������һ�εĺϽ�ȫ���μӷ�Ӧ�����ɵ�����������Ϊ��0.56L��0.0893g/L��0.05g
��þ������Ϊx�����ɵ�����������Ϊa������������Ϊ0.51g-x�����ɵ�����������Ϊ0.05g-a
Mg+2HCl�TMgCl2+H2��
24 2
x a
$\frac{24}{x}=\frac{2}{a}$
2Al+6HCl�T2AlCl3+3H2��
54 6
0.51g-x 0.05g-a
$\frac{54}{0.51g-x}=\frac{6}{0.05g-a}$
���x=0.24g��
����������Ϊ0.51g-0.24g=0.27g
������þ��������Ϊ��0.27g��0.24g=9��8
�𣺺Ͻ�����þ��������Ϊ9��8��
���� ������Ҫ����ѧ�����û�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Թ� | B�� | �ձ� | C�� | ��ƿ | D�� | ��ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | a | b | c | d |
| ��Ӧǰ��������g�� | 6.4 | 1.0 | 0.1 | 1.6 |
| tʱ�̵�������g�� | 3.2 | X | ||
| ��Ӧ���������g�� | 0 | 5.4 | Y | 0 |
| A�� | a��c�Ƿ�Ӧ�� | |
| B�� | X=2g | |
| C�� | �÷�Ӧ�ǻ����� | |
| D�� | �÷�Ӧ��ѧ����ʽ��a��d�Ļ�ѧ������֮��Ϊ2��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 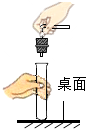 �������� | B�� | 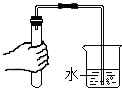 ��������� | C�� | 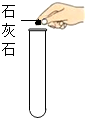 ȡ��ʯ��ʯ | D�� | 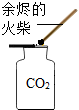 CO2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
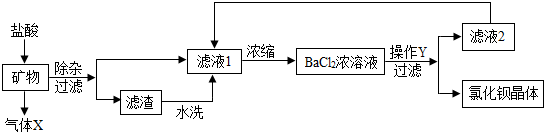
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ɵ�����һ�������� | |
| B�� | ���ӿɷ�ԭ��һ�����ɷ� | |
| C�� | ����һ���ܱ������ʵĻ�ѧ���� | |
| D�� | Ԫ�ص�����һ����ԭ�ӵ��������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ľ̿��������ȼ�գ����ɴ̼�����ζ������ | |
| B�� | �����ڿ�����ȼ�ղ��������İ��� | |
| C�� | ��˿�������о���ȼ�գ��������䣬���ɺ�ɫ���� | |
| D�� | һ����̼��ԭ����ͭʱ���������ʵ���ɫ�ɺ�ɫ��Ϊ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
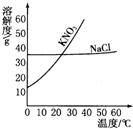 ������ͼ��ʾ���ܽ�����ߣ��ش��������⣮
������ͼ��ʾ���ܽ�����ߣ��ش��������⣮�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com