
| Ėł¼ÓŃĪĖįµÄ“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī | µŚĖÄ“Ī |
| ŃĪĖįµÄÖŹĮæ/g | 25 | 25 | 25 | 25 |
| ÉÕ±¼°ĖłŹ¢ĪļÖŹ×ÜÖŹĮæ/g | 181.2 | 204.4 | 228.6 | 253.6 |
| 106 |
| x |
| 44 |
| 4.4g |
| 10.6g |
| 12g |
| 73 |
| y |
| 44 |
| 1.8g |
| 131.4 |
| 44 |
| ||
| 25g |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| Ėł¼ÓŃĪĖįµÄ“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī | µŚĖÄ“Ī |
| ŃĪĖįµÄÖŹĮæ/g | 25 | 25 | 25 | 25 |
| ÉÕ±¼°ĖłŹ¢ĪļÖŹ×ÜÖŹĮæ/g | 181.2 | 204.4 | 228.6 | 253.6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| Ėł¼ÓŃĪĖįµÄ“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī | µŚĖÄ“Ī |
| ŃĪĖįµÄÖŹĮæ/g | 25 | 25 | 25 | 25 |
| ÉÕ±¼°ĖłŹ¢ĪļÖŹ×ÜÖŹĮæ/g | 181.2 | 204.4 | 228.6 | 253.6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012½ģĖÄ“ØŹ”ŅĖ±öŹŠÖŠæ¼Ä£Äā»ÆѧŹŌ¾ķ ĢāŠĶ£ŗ¼ĘĖćĢā
£Ø3·Ö£©Ä³»Æ¹¤³§ÓĆ°±¼ī·ØÉś²śµÄ“æ¼ī²śĘ·ÖŠŗ¬ÓŠÉŁĮæĀČ»ÆÄĘŌÓÖŹ£¬Ęä²śĘ·°ü×°“üÉĻ×¢Ć÷£ŗĢ¼ĖįÄĘ”Ż96%”£ĪŖ²ā¶ØøĆ²śĘ·ÖŠŗ¬Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬½ųŠŠĮĖŅŌĻĀŹµŃé£ŗČ”12g“æ¼īѳʷ·ÅČėÉÕ±ÖŠ£¬³ĘµĆÉÕ±¼°“æ¼īѳʷµÄ×ÜÖŹĮæĪŖ158g£¬ŌŁ°Ń100gĻ”ŃĪĖįĘ½¾ł·Ö³ÉĖÄ·Ż£¬ŅĄ“Ī¼ÓČėµ½ŃłĘ·ÖŠ£¬Ćæ“Ī¾ł³ä·Ö·“Ó¦”£ŹµŃ鏿¾Ż¼ĒĀ¼ČēĻĀ£ŗ
| Ėł¼ÓŃĪĖįµÄ“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī | µŚĖÄ“Ī |
| ŃĪĖįµÄÖŹĮæ/g | 25 | 25 | 25 | 25 |
| ÉÕ±¼°ĖłŹ¢ĪļÖŹ×ÜÖŹĮæ/g | 181.2 | 204.4 | 228.6 | 253.6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij»Æ¹¤³§ÓĆ°±¼ī·ØÉś²śµÄ“æ¼ī²śĘ·ÖŠŗ¬ÓŠÉŁĮæĀČ»ÆÄĘŌÓÖŹ£¬Ęä²śĘ·°ü×°“üÉĻ×¢Ć÷£ŗĢ¼ĖįÄĘ=96£„”£ĪŖ²ā¶ØøĆ²śĘ·ÖŠŗ¬Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬½ųŠŠĮĖŅŌĻĀŹµŃé£ŗČ”11.0g“æ¼īѳʷ·ÅČėÉÕ±ÖŠ£¬³ĘµĆÉÕ±¼°ĖłŹ¢“æ¼īѳʷµÄ×ÜÖŹĮæĪŖ158.0g£¬ŌŁ°Ń100gĻ”ŃĪĖįĘ½¾ł·Ö³ÉĖÄ·ŻŅĄ“Ī¼ÓČėѳʷ֊£¬Ćæ“Ī¾ł³ä·Ö·“Ó¦”£ŹµŃ鏿¾Ż¼ĒĀ¼ČēĻĀ£ŗ
| Ėł¼ÓŃĪĖįµÄ“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚ¶ž“Ī | µŚĖÄ“Ī |
| ÉÕ±¼°ĖłŹ¢ĪļÖŹ×ÜÖŹĮæ/g | 181.2 | 204.4 | 228.6 | 253.6 |
ĒėÄć¾Ż“Ė·ÖĪö¼ĘĖć£ŗ
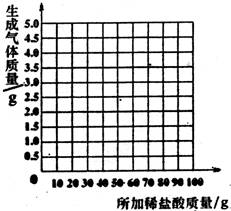
£Ø1£©µŚŅ»“Ī¼ÓČėĻ”ŃĪĖį³ä·Ö·“Ó¦ŗó£¬Éś³É¶žŃõ»ÆĢ¼µÄÖŹĮæŹĒ g”£
£Ø2£©øĆ²śĘ·ÖŠĢ¼ĖįÄʵÄÖŹĮæ·ÖŹżŹĒ·ńŗĻøń£æ£ØŅŖĒ󊓳ö¼ĘĖć¹ż³Ģ£¬½į¹ū¾«Č·µ½0.1£„£©
£Ø3£©øł¾ŻŹµŃ鏿¾Ż£¬ŌŚĻĀ±ßµÄ×ų±źÖ½ÉĻ»ęÖĘ³öĖł¼ÓĻ”ŃĪĖįÖŹĮæÓėÉś³ÉĘųĢåÖŹĮæ¹ŲĻµµÄĒśĻß”££Ø²»ŅŖĒóĮŠ¼ĘĖć¹ż³Ģ£¬Ö»»³öĒśĻß¼“æÉ£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com