
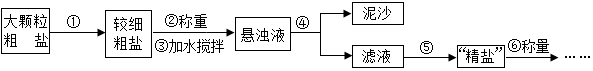
���� ��1�����ݹ��˵�ע����������жϣ�
��2�����ݾ����Ƶ���=$\frac{���ε�����}{���ε�����}$��100%�������
��� �⣺��1�����˺�õ�����Һ��Ȼ���ǣ���������ֽ�����Һ�������ֽ��Ե��ʹҺ�岻������ֱ�������ձ��ȣ�
��2�������Ƶ���=$\frac{���ε�����}{���ε�����}$��100%����Ȼ���εIJ��ʵͣ�˵�����ᴿʱ��ʧ�˾��Σ�
A��ʳ��û����ȫ�ܽ⣬��ʹ���ε��������٣���A��ȷ��
B��ʳ�ηɽ��˵����˾��εļ��٣���B��ȷ��
C�����κܳ������˾��ε���������ʹ������������C����
D���������еľ���û��ȫ��ת�Ƶ�����ֽ�ϣ���ʹ���ε�������С����D��ȷ��
�ʴ�Ϊ����1����ֽ��������ʱҺ�������ֽ�ı�Ե����2��ABD��
���� �����ۺϿ����˴����ᴿʵ����й�ע��������ʱҪ�������֪ʶϸ�ķ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ��Ŀ�� | �������� |
| A | ��ȥCO2�е�����HC1���� | ��ͨ����������������Һ����ͨ��Ũ���� |
| B | ��ȥ�Ȼ��ع����е������������� | ��������ˮ�ܽ⣬���� |
| C | ��ȥKNO3��Һ�е���K2SO4 | ���������Ba��NO3��2��Һ������ |
| D | ��ȥͭ���е���������ͭ | �������ϡ���ᣬ���ˣ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ӧ��ȡ��ˮ�����Ϊ180mL | |
| B�� | ʵ������Ҫ�����������ձ�����Ͳ�������� | |
| C�� | װƿʱ��С����©һ������Һ����ƿ��������ҺŨ�ȵ���10% | |
| D�� | �����ڱڸ���ˮ����ձ�������Һ����������Һ����������С��10% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ͼΪij��ʯ��Ʒ1�����У�����Ԫ�غ����ı���ͼ������Ʒ�в����ܺ������к��ֻ������������
ͼΪij��ʯ��Ʒ1�����У�����Ԫ�غ����ı���ͼ������Ʒ�в����ܺ������к��ֻ������������| A�� | ̼��� | B�� | ����� | C�� | ������ | D�� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼΪʵ������ijŨ�����Լ�ƿ��ǩ�ϵIJ�����Ϣ���Իش���������
��ͼΪʵ������ijŨ�����Լ�ƿ��ǩ�ϵIJ�����Ϣ���Իش����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com