
| ���� | NaHCO3 | CH3COONa | NaClO | NaCN | Na2CO3 |
| pH | 8.6 | 8.8 | 10.3 | 11.1 | 11.6 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | CH3COOH | NaHCO3 | Na2CO3 | NaClO | NaCN | NaCl |
| pH | 8.8 | 8.6 | 11.6 | 10.3 | 11.1 | 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
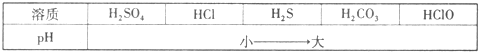
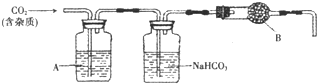
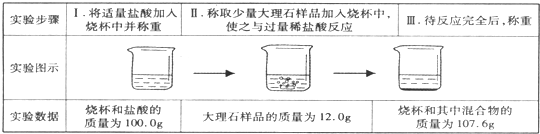
 CaCO3�㷺��������Ȼ�磬��һ����Ҫ�Ļ���ԭ�ϣ�ij�ִ���ʯ����Ҫ�ɷ�ΪCaCO3�⣬���������������С���С��ͬѧ�����ִ���ʯ��ϡ���ᷴӦ���ֱ�չ����̽�����������̽�����ش�������⣮
CaCO3�㷺��������Ȼ�磬��һ����Ҫ�Ļ���ԭ�ϣ�ij�ִ���ʯ����Ҫ�ɷ�ΪCaCO3�⣬���������������С���С��ͬѧ�����ִ���ʯ��ϡ���ᷴӦ���ֱ�չ����̽�����������̽�����ش�������⣮| ���� | H2SO4 | HCl | H2S | H2CO3 | HClO |
| pH | ���� | ||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪���ֽⷴӦ2CH3COOH+Na2CO3=2CH3COONa+H2O+CO2���ɽ��У��ڳ����£������ͬŨ�ȵ�����������Һ��pH��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ���� | NaHCO3 | CH3COONa | NaClO | NaCN | Na2CO3 |
| pH | 8.6 | 8.8 | 10.3 | 11.1 | 11.6 |
| A��CO2+H2O+2NaClO=Na2CO3+2HClO |
| B��CO2+H2O+NaClO=NaHCO3+HClO |
| C��CH3COOH+NaCN=CH3COONa+HCN |
| D��NaClO+CH3COOH=HClO+CH3COONa |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com