
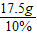 =175g£¬ĖłŅŌŠčŅŖĖ®ÖŹĮæĪŖ£ŗ175g-17.5g=157.5g£¬ĖłŅŌĖ®µÄĢå»żĪŖ£ŗ157.5mL£»
=175g£¬ĖłŅŌŠčŅŖĖ®ÖŹĮæĪŖ£ŗ175g-17.5g=157.5g£¬ĖłŅŌĖ®µÄĢå»żĪŖ£ŗ157.5mL£»

 Š”Ģģ²ÅæĪŹ±×÷ŅµĻµĮŠ“š°ø
Š”Ģģ²ÅæĪŹ±×÷ŅµĻµĮŠ“š°ø Ņ»æĪĖÄĮ·ĻµĮŠ“š°ø
Ņ»æĪĖÄĮ·ĻµĮŠ“š°ø »ĘøŌŠ”דŌŖĀś·Ö³å“ĢĪ¢²āŃéĻµĮŠ“š°ø
»ĘøŌŠ”דŌŖĀś·Ö³å“ĢĪ¢²āŃéĻµĮŠ“š°ø ŠĀøؽĢµ¼Ń§ĻµĮŠ“š°ø
ŠĀøؽĢµ¼Ń§ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğĢģ½ņŹŠ“óøŪÓĶĢļ֊漶žÄ£»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ¼ņ“šĢā
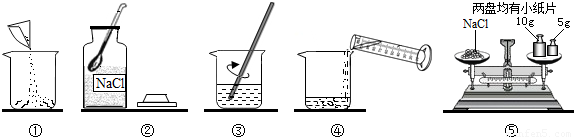
ŹµŃéŹŅ³£ÅäÖĘŅ»¶ØČÜÖŹÖŹĮæ·ÖŹżµÄČÜŅŗ½ųŠŠŹµŃ锣Š”ŗ£Ķ¬Ń§×¼±øÅäÖĘ10%µÄNaClČÜŅŗ£¬ĻĀĶ¼ŹĒÅäÖʵďµŃé²Ł×÷Ź¾ŅāĶ¼”£
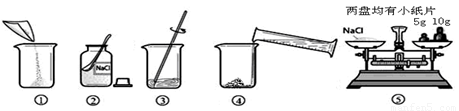
£Ø1£©ÉĻĶ¼µÄŠņŗűķŹ¾Š”ŗ£ÅäÖĘČÜŅŗµÄ²Ł×÷Ė³Šņ____________________”£
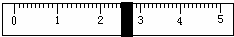
£Ø2£©³ĘĮæNaClŹ±£¬ĢģĘ½Ę½ŗāŗóµÄדĢ¬ČēĶ¼¢ŻĖłŹ¾£¬ÓĪĀė±ź³ßŹ¾Źż¼ūĻĀĶ¼£¬Ōņ³ĘČ”µÄNaClÖŹĮæĪŖ___________”£

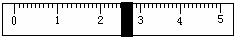
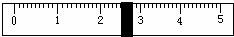
£Ø3£©øł¾Ż¼ĘĖćŠčŅŖĮæČ”Ė®µÄĢå»żŹĒ___________ mL£ØĖ®µÄĆܶČĪŖ1g/mL£©”£
£Ø4£©ĮæČ”Ė®µÄĢå»żŹ±£¬Š”ĮĮŃöŹÓ¶ĮŹż£¬ŌņĖłÅäČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹż_________£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©10%”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
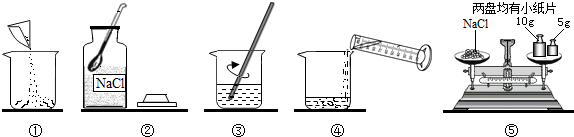
ŹµŃéŹŅ³£ÅäÖĘŅ»¶ØČÜÖŹÖŹĮæ·ÖŹżµÄČÜŅŗ½ųŠŠŹµŃ锣Š”ŗ£Ķ¬Ń§×¼±øÅäÖĘ10%µÄNaClČÜŅŗ£¬ĻĀĶ¼ŹĒÅäÖʵďµŃé²Ł×÷Ź¾ŅāĶ¼”£
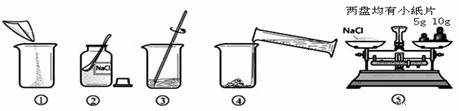
£Ø1£©ÉĻĶ¼µÄŠņŗűķŹ¾Š”ŗ£ÅäÖĘČÜŅŗµÄ²Ł×÷Ė³Šņ____________________”£
£Ø2£©³ĘĮæNaClŹ±£¬ĢģĘ½Ę½ŗāŗóµÄדĢ¬ČēĶ¼¢ŻĖłŹ¾£¬ÓĪĀė±ź³ßŹ¾Źż¼ūĻĀĶ¼£¬Ōņ³ĘČ”µÄNaClÖŹĮæĪŖ___________”£
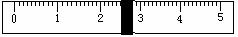
£Ø3£©øł¾Ż¼ĘĖćŠčŅŖĮæČ”Ė®µÄĢå»żŹĒ___________ mL£ØĖ®µÄĆܶČĪŖ1g/mL£©”£
£Ø4£©ĮæČ”Ė®µÄĢå»żŹ±£¬Š”ĮĮŃöŹÓ¶ĮŹż£¬ŌņĖłÅäČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹż_________£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©10%”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com