
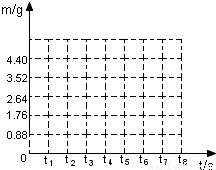
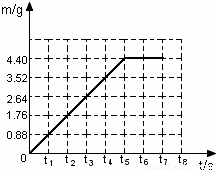
| ��Ӧʱ��t/s | t | t1 | t2 | t3 | t4 | t5 | t6 |
| ��������m/g | 0.88 | 1.76 | 2.64 | 3.52 | 4.4 | 4.4 |
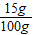 ×100%=15%
×100%=15%


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п���ѧ����������7���Ӻ�ԭ�� ���ͣ�ѡ����
��2011���Ĵ����ݣ�13�⣩�ڵ����ľ�Ԯ�ж��У�Ϊ���������ڷ����е��Ҵ��ߣ����������Ѿ�Ȯ���Ѿ�Ȯ�ܸ������巢������ζ�����Ҵ��ߡ����û�ѧ�۵�����ĽǶȷ����ܷ����Ҵ��ߵ�ԭ���ǣ� ��
A�����Ӻ�С B�������ڲ�ͣ���˶�
C�����Ӽ��м�϶ D����������ԭ�ӹ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���Ĵ�ʡ�������п���ѧ�Ծ��������棩 ���ͣ�ѡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����Ӻ�С | B�������ڲ�ͣ���˶� |
| C�����Ӽ��м�϶ | D����������ԭ�ӹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п���ѧ����������7 ���Ӻ�ԭ�� ���ͣ���ѡ��
��2011���Ĵ����ݣ�13�⣩�ڵ����ľ�Ԯ�ж��У�Ϊ���������ڷ����е��Ҵ��ߣ����������Ѿ�Ȯ���Ѿ�Ȯ�ܸ������巢������ζ�����Ҵ��ߡ����û�ѧ�۵�����ĽǶȷ����ܷ����Ҵ��ߵ�ԭ���ǣ� ��
| A�����Ӻ�С | B�������ڲ�ͣ���˶� |
| C�����Ӽ��м�϶ | D����������ԭ�ӹ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com