
½«COŗĶCO2µÄ»ģŗĻĘųĢå¹²3.2gĶعż×ćĮæ×ĘČȵÄŃõ»ÆĶ·ŪÄ©£¬³ä·Ö·“Ó¦ŗ󣬽«ĘųĢåĶØČė×ćĮæ³ĪĒåŹÆ»ŅĖ®ÖŠ£ØĘųĢåČ«²æ±»ĪüŹÕ£©£¬¹żĀĖ£¬²āµĆČÜŅŗÖŹĮæ¼õÉŁ5.6g £¬ŌņŌ»ģŗĻĘųĢåÖŠĢ¼ŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ£Ø”” ””£©”£
A. 21.0% B. 27.3% C. 33.3% D. 37.5%
D ”¾½āĪö”æÉč·“Ó¦ŗóÉś³É¶žŃõ»ÆĢ¼µÄ×ÜÖŹĮæĪŖx CO2 + Ca(OH)2 == CaCO3 ”ż+ H2O ČÜŅŗ¼õÉŁµÄÖŹĮæ 44 100-44 X 5.6g = x=4.4g 4.4g¶žŃõ»ÆĢ¼ÖŠĢ¼ŌŖĖŲµÄÖŹĮæĪŖ=1.2g ŌņŌ»ģŗĻĘųĢåÖŠĢ¼ŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ=37.5%£¬¹ŹŃ”D”£ ĆūĢā½š¾ķĻµĮŠ“š°ø
ĆūĢā½š¾ķĻµĮŠ“š°ø ÓżӾ«¾ķĻµĮŠ“š°ø
ÓżӾ«¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ½Ī÷Ź”øÓÖŻŹŠŠÄ³ĒĒųĮłŠ£ĮŖĆĖ2018½ģ¾ÅÄź¼¶ĻĀѧʌĮŖæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠĪļÖŹÖŠ£¬ŹōÓŚ“æ¾»ĪļµÄŹĒ£Ø £©
A. ÉśĄķŃĪĖ® B. øßĆĢĖį¼Ų C. ø»ŃõæÕĘų D. ŅŅ“¼ĘūÓĶ
B ”¾½āĪö”æA”¢ÉśĄķŃĪĖ®ÖŠÓŠŹ³ŃĪ”¢Ė®£¬ŹōÓŚ»ģŗĻĪļ£¬“ķĪó£»B”¢øßĆĢĖį¼ŲŹĒŅ»ÖÖĪļÖŹ£¬ŹōÓŚ“æ¾»Īļ£¬ÕżČ·£»C”¢ø»ŃõæÕĘųæÕĘųÖŠÓŠŃõĘų”¢µŖĘųµČĪļÖŹ£¬ŹōÓŚ»ģŗĻĪļ£¬“ķĪó£»D”¢ŅŅ“¼ĘūÓĶÖŠÓŠŅŅ“¼”¢ĘūÓĶµČĪļÖŹ£¬ŹōÓŚ»ģŗĻĪļ£¬“ķĪ󔣲éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ½ĖÕŹ”2018½ģ¾ÅÄź¼¶3ŌĀÄ£Äāæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
øł¾ŻĶ¼Ź¾ŹµŃé×°ÖĆ£¬»Ų“šĻĀĮŠĪŹĢā”£

(1)Ķ¼ÖŠaŅĒĘ÷µÄĆū³Ę£ŗa________________£¬b_____”£
(2)ÓĆøßĆĢĖį¼Ų¹ĢĢåÖĘŃõĘų£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ___________________________”£½«×°ÖĆAŗĶDĮ¬½Ó½ųŠŠ“ĖŹµŃ飬ŹµŃé½įŹų£¬Ķ£Ö¹¼ÓČČĒ°ŅŖĻČ______£¬ÄæµÄŹĒ______”£
(3)Š“³öŹµŃéŹŅÖĘČ”¶žŃõ»ÆĢ¼µÄ»Æѧ·½³ĢŹ½________________£¬Ęä·¢Éś×°ÖĆæÉÓĆC(¶ąæ×øō°åÓĆĄ“·Åæéד¹ĢĢå)“śĢęBµÄÓŵćŹĒ________________£¬ČēÓĆE×°ÖĆŹÕ¼ÆCO2£¬ŌņĘųĢåÓ¦“Ó____________¶ĖĶØČė(Ģī”°c”±»ņ”°d”±)”£
¾Ę¾«µĘ ³¤¾±Ā©¶· 2KMnO4K2MnO4+MnO2+O2”ü ½«µ¼¹ÜŅĘ³öĖ®Ćę ·ĄÖ¹Ė®µ¹ĪüŌģ³ÉŹŌ¹ÜÕØĮŃ CaCO3+2HClØTCaCl2+H2O+CO2”ü æŲÖĘ·“Ó¦µÄ·¢ÉśÓėĶ£Ö¹£¬½ŚŌ¼Ņ©Ę· c ”¾½āĪö”æ±¾ĢāÖ÷ŅŖæ¼²éĮĖŅĒĘ÷µÄĆū³Ę”¢ĘųĢåµÄÖĘČ”×°ÖĆŗĶŹÕ¼Æ×°ÖƵÄŃ”Ōń£¬Ķ¬Ź±Ņ²æ¼²éĮĖ»Æѧ·½³ĢŹ½µÄŹéŠ“ŌÓµČ£¬×ŪŗĻŠŌ±Č½ĻĒ森ĘųĢåµÄÖĘČ”×°ÖƵÄŃ”ŌńÓė·“Ó¦ĪļµÄדĢ¬ŗĶ·“Ó¦µÄĢõ¼žÓŠ¹Ų£»ĘųĢåµÄŹÕ¼Æ×°ÖƵÄŃ”ŌńÓėĘųĢåµÄĆܶČŗĶČܽā...²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ½ĖÕŹ”2018½ģ¾ÅÄź¼¶3ŌĀÄ£Äāæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗµ„Ń”Ģā
ČĖĢåÄŚŌŖĖŲŹ§ŗāŹĒÖŲŅŖµÄÖĀ²”ŅņĖŲ”£ĻĀĮŠ¼²²”æÉÄÜÓėȱøĘÓŠ¹ŲµÄŹĒ
A”¢ŲžŁĶ²” B”¢“ó²±×Ó²” C”¢Ę¶ŃŖÖ¢ D”¢ÖĒĮ¦µĶĻĀ
A ”¾½āĪö”æ ŹŌĢā·ÖĪö£ŗČĖĢåÄŚŌŖĖŲŹ§ŗāŹĒÖŲŅŖµÄÖĀ²”ŅņĖŲ”£Č±øĘ»įŅżĘšŲžŁĶ²””£¹ŹŃ”A.²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗɽ¶«Ź”2018½ģ¾ÅÄź¼¶Ņ»Ä£»ÆѧŹŌ¾ķ ĢāŠĶ£ŗ×ŪŗĻĢā
½šŹō²ÄĮĻ¾ßÓŠÓÅĮ¼µÄŠŌÄÜ£¬±»¹ć·ŗÓ¦ÓĆÓŚÉś²ś”¢Éś»īÖŠ”£
¢ÅĻĀĮŠ½šŹōÖĘĘ·ÖŠ£¬Ö÷ŅŖĄūÓĆĮĖ½šŹōµ¼µēŠŌµÄŹĒ_________”££ØĢīŠņŗÅ£©
A£®»Ę½šŹĪĘ· B£®Ģś¹ų C£®Ķµ¼Ļß D£®²»ŠāøÖµ¶¾ß
¢ĘøÖĢśŠāŹ“»įŌģ³ÉŃĻÖŲµÄ׏Ō“ĄĖ·Ń£¬·ĄÖ¹»ņ¼õ»ŗøÖĢśŠāŹ“µÄ³£ÓĆ·½·ØÓŠ__________”££ØŠ“³öŅ»Ģõ¼“æÉ£©
¢Ē”°ŌųĒąµĆĢśŌņ»ÆĪŖĶ”±£¬ÕāŹĒŹĄ½ēŹŖ·ØŅ±½šµÄĻČĒż”£ŹŌŠ“³öÓĆĢśŗĶĮņĖįĶČÜŅŗĪŖŌĮĻ½ųŠŠŹŖ·ØĮ¶ĶµÄ»Æѧ·½³ĢŹ½____________£¬ĖüŹōÓŚ__________ ·“Ó¦”££ØĢī”°»ÆŗĻ”±”¢”°·Ö½ā”±”¢”°ø“·Ö½ā”±”¢”°ÖĆ»»”±Ö®Ņ»£©
¢ČĪŖĮĖ²ā¶Øij»ĘĶ£ØĶŠæŗĻ½š£©ŃłĘ·µÄ×é³É£¬Ä³ŃŠ¾æŠŌѧĻ°Š”×é³ĘČ”ĮĖøĆѳʷ20g£¬ĻņĘäÖŠÖšµĪ¼ÓČė9.8%µÄĻ”ĮņĖįÖĮøÕŗĆ²»ŌŁ²śÉśĘųĢåĪŖÖ¹”£·“Ó¦¹ż³ĢÖŠ£¬Éś³ÉĘųĢåÓėĖłÓĆĮņĖįČÜŅŗµÄÖŹĮæ¹ŲĻµČēĻĀĶ¼ĖłŹ¾”£ŹŌ¼ĘĖć£ŗøĆ»ĘĶѳʷ֊ĶµÄÖŹĮæĪŖ______________£æ
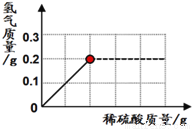
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗɽ¶«Ź”2018½ģ¾ÅÄź¼¶Ņ»Ä£»ÆѧŹŌ¾ķ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠÓŠ¹ŲĒāŃõ»ÆÄĘŠŌÖŹĢ½¾æŹµŃéµÄŠšŹöÖŠ£¬“ķĪóµÄŹĒ£Ø £©”£

A. ¼×Ļņ½Ó½ü·ŠĢŚµÄĖ®ÖŠ¼ÓČėŅ»¶ØĮæNaOH¹ĢĢ壬ŅŗĢåŃøĖŁ·ŠĢŚ
B. ŅŅ½«ÉŁĮæĶ··¢¼ÓČėµ½ČȵÄÅØNaOHČÜŅŗÖŠ£¬Ķ··¢Öš½„ČܽāĻūŹ§
C. ±ūĻņ¾ĆÖĆæÕĘųĄļµÄĒāŃõ»ÆÄĘČÜŅŗÖŠµĪ¼ÓĪŽÉ«·ÓĢŖŹŌŅŗ£¬¼ģŃéĘäŹĒ·ń±äÖŹ
D. ¶”ĻņŹ¢ĀśCO2ĘųĢåµÄ¼ÆĘųĘæÖŠ¼ÓČėŹŹĮæÅØÉÕ¼īČÜŅŗ£¬¼¦µ°±»”°ĶĢ”±ČėĘæÖŠ
C ”¾½āĪö”æA”¢ĒāŃõ»ÆÄĘČÜÓŚĖ®·Å³öČČĮ棬¹ŹĻņ½Ó½ü·ŠĢŚµÄĖ®ÖŠ¼ÓČėŅ»¶ØĮæNaOH¹ĢĢ壬ŅŗĢåŃøĖŁ·ŠĢŚ£¬ÕżČ·£» B”¢Ķ··¢ŹĒµ°°×ÖŹ£¬ÓėČȵÄĒāŃõ»ÆÄĘČÜÓŚ·“Ó¦£¬¹ŹĶ··¢Öš½„ČܽāĻūŹ§£¬ÕżČ·£» C”¢ĒāŃõ»ÆÄʱäÖŹŗóÉś³ÉµÄĢ¼ĖįÄĘČÜŅŗČŌČ»ĻŌ¼īŠŌ£¬¹Ź²»ÄÜÓĆĪŽÉ«·ÓĢŖŹŌŅŗ£¬¼ģŃéĘäŹĒ·ń±äÖŹ£¬“ķĪó£» D”¢ĻņŹ¢ĀśCO2ĘųĢåµÄ¼ÆĘųĘæÖŠ¼ÓČėŹŹĮæÅØÉÕ¼īČÜŅŗ£¬ĒāŃõ»ÆÄĘÓė¶žŃõ»ÆĢ¼·“Ó¦£¬ĘæÄŚŃ¹Ēæ½µµĶ£¬ŌŚ“óĘųŃ¹µÄ×÷ÓĆĻĀ£¬¼¦µ°...²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗɽ¶«Ź”2018½ģ¾ÅÄź¼¶Ņ»Ä£»ÆѧŹŌ¾ķ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĶ¼ĪŖ³õÖŠ»ÆѧŹµŃéæ¼²éÖŠ³£¼ūµÄ²æ·Ö²Ł×÷£¬ĘäÖŠÕżČ·µÄŹĒ£Ø”” ””£©”£

A. A B. B C. C D. D
D ”¾½āĪö”æA”¢²ā¶ØČÜŅŗµÄpHŹ±²»Äܽ«pHŹŌÖ½Ö±½Ó²åČė“ż²āŅŗĢåÖŠ£¬“ķĪó£» B”¢ĻņŹŌ¹ÜÖŠ¼ÓČė¹ĢĢåĢś¶¤Ź±£¬ŅŖ½«ŹŌ¹ÜĘ½·Å£¬“ķĪó£» C”¢øĆ¹żĀĖ×°ÖĆ֊ȱɣ²£Į§°ō£¬“ķĪó£» D”¢¼ģ²é×°ÖƵÄĘųĆÜŠŌµÄ·½·ØŹĒÕżČ·µÄ”£¹ŹŃ”D”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗÖŲĒģŹŠ£Ø½½ņ¶žÖŠµČ£©°ĖŠ£2018½ģ¾ÅÄź¼¶ĻĀѧʌµŚŅ»½×¶Īæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗµ„Ń”Ģā
ÓĆŹŹµ±µÄ·½·Ø°ŃĻõĖį¼ŲµÄ²»±„ŗĶČÜŅŗ×Ŗ±äĪŖ±„ŗĶČÜŅŗ,ĘäČÜŅŗÖŹĮæ( )
A. ±ä“ó B. ±äŠ” C. ²»±ä D. ±ä“󔢱䊔”¢²»±ä¶¼ÓŠæÉÄÜ
D ”¾½āĪö”潫ĻõĖį¼ŲµÄ²»±„ŗĶČÜŅŗ±äĪŖ±„ŗĶČÜŅŗµÄ·½·ØÓŠ¼ÓČėĻõĖį¼Ų£¬¼õÉŁĖ®£¬½µµĶĪĀ¶Č£¬µ±¼ÓČėĻõĖį¼ŲŹ±£¬ČÜŅŗµÄÖŹĮæŌö¼Ó£¬µ±¼õÉŁĖ®Ź±£¬ČÜŅŗµÄÖŹĮæ¼õŠ”£¬µ±½µµĶĪĀ¶ČŹ±£¬ČÜŅŗµÄÖŹĮæ²»±ä£¬¹ŹŃ”D”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗŗÓ±±Ź”2018½ģ¾ÅÄź¼¶ÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗµ„Ń”Ģā
ŌŚÉś²śÉś»īÖŠ³£ÓƵ½ĻĀĮŠĪļÖŹ£¬ŹōÓŚ“æ¾»ĪļµÄŹĒ
A. ŹÆ»ŅĖ® B. ²»ŠāøÖ C. øɱł D. ŃĄøą
C ”¾½āĪö”æA”¢ŹÆ»ŅĖ®ÖŠŗ¬ÓŠĖ®”¢ĒāŃõ»ÆøʵČĪļÖŹ£¬ŹōÓŚ»ģŗĻĪļ£®¹ŹŃ”Ļī“ķĪó£» B”¢²»ŠāøÖŹĒĢśµÄŗĻ½š£¬²»ŠāøÖÖŠ³żŗ¬ĢśĶā£¬»¹ÓŠC”¢Cr”¢Ni µČ£¬ŹōÓŚ»ģŗĻĪļ£¬¹ŹŃ”Ļī“ķĪó£» C”¢øɱłÖ»ŗ¬ÓŠŅ»ÖÖĪļÖŹ£¬ŹōÓŚ“æ¾»Īļ£®¹ŹŃ”ĻīÕżČ·£» D”¢ŃĄøąÖŠŗ¬ÓŠÄ¦²Į¼Į£ØĢ¼ĖįøĘµČ£©”¢±£ŹŖ¼Į£ØøŹÓĶµČ£©”¢ĢšĪ¶¼ĮµČ¶ąÖÖĪļÖŹ£¬ŹōÓŚ»ģŗĻĪļ£¬¹ŹŃ”Ļī“ķĪó£® ¹ŹŃ”C£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com