
| �� �� | ��1�� | ��2�� | ��3�� |
| ��ȡ������Ʒ������/g | 7 | 5 | 5 |
| �����Ȼ�����Һ������/g | 50 | 50 | 75 |
| ��Ӧ�����ɳ���������/g | 4 | 4 | 4 |
���� ��1����һ�κ͵ڶ�����ȡ�Ĺ����������ȣ�������ȡ�Ȼ�����Һ��������ȣ��������ɵij�����������ȣ����Կ����жϵ�һ����ȡ������ʣ�࣬��5g����Ʒ��Ӧ�������ɳ���������Ϊ4g����2�κ͵�3����ȡ�Ĺ�����Ʒ��ȣ�������ȡ���Ȼ�����Һ���������ȣ��������ɵij�����������ȣ����Կ����жϵ�3����ȡ���Ȼ�����Һ��ʣ�࣬��˵��50g�Ȼ�����Һ��Ӧ��ֻ�ܵõ�4g���������Ϸ�������֪���ڶ���������������ǡ����ȫ��Ӧ�����Կ��Ը��ݵ�2�η�Ӧ�����ݽ��н�𣻸������ɳ������������Լ����̼���Ƶ�������
��2���������ɳ������������Լ�����Ȼ��Ƶ�����������������Ȼ�����Һ������������
��3�����ݣ�1��������̼���Ƶ��������Լ�����������Ȼ��Ƶ����������Ծݴ˽����⣮
��� �⣺��1�����������������ʵ�����ݿ�֪����һ�κ͵ڶ�����ȡ�Ĺ����������ȣ�������ȡ�Ȼ�����Һ��������ȣ��������ɵij�����������ȣ����Կ����жϵ�һ����ȡ������ʣ�࣬��5g����Ʒ��Ӧ�������ɳ���������Ϊ4g����2�κ͵�3����ȡ�Ĺ�����Ʒ��ȣ�������ȡ���Ȼ�����Һ���������ȣ��������ɵij�����������ȣ����Կ����жϵ�3����ȡ���Ȼ�����Һ��ʣ�࣬��˵��50g�Ȼ�����Һ��Ӧ��ֻ�ܵõ�4g���������Ϸ�������֪���ڶ���������������ǡ����ȫ��Ӧ��
��̼���Ƶ�����Ϊx���μӷ�Ӧ���Ȼ�������Ϊy
Na2CO3+CaCl2�TCaCO3��+2NaCl
106 111 100
x y 4g
$\frac{106}{x}=\frac{111}{y}=\frac{100}{4g}$
��ã�x=4.24g��y=4.44 g
��2��������Ȼ�����Һ��������������Ϊ��$\frac{11.1g}{50g}��$100%=8.88%��
��3��̼������Ʒ�������Ȼ��Ƶ�������5g-4.24g=0.76g��
̼������Ʒ�������Ȼ��Ƶ�����������$\frac{0.75g}{5g}$��100%=15.2%��
�𰸣�
��1��5g̼������Ʒ��̼���Ƶ�������4.24g��
��2�������Ȼ�����Һ����������������8.88%��
��3��̼������Ʒ�������Ȼ��Ƶ�����������15.2%
���� �����ǽ�������ѧģ�ͣ�����ͼ���ķ�ʽ�������ͽ����ѧ�����е��й����⣬Ҫ��ѧ���н�ǿ��ʶͼ���������ݷ���������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CuSO4��NaOH��KNO3��NaCl | B�� | K2CO3��NaOH��ϡHCl��BaCl2 | ||
| C�� | FeCl3��KOH��ϡHCl��ϡH2SO4 | D�� | ϡHCl��Na2CO3��ϡH2SO4��Na2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����Ķ����糿��ͬѧ��С��һ����ů��������С�ɿɰ���ͬѧ�������������ů���������ü��ȣ�ֻҪ��װ����һ������ܸ�����ů�֣���ҷdz����棬��һ�ԣ�������ˣ�ͬѧ�Dz����ԡ�ů���������ڲ��ɷֲ����˼���ĺ��棬��ֽ����ԭ����һЩ����ɫ��ĩ������һ�������ð�̣������֣���ô����Щ��ɫ��ĩ�ɷ��ֻ���ʲô�أ�
����Ķ����糿��ͬѧ��С��һ����ů��������С�ɿɰ���ͬѧ�������������ů���������ü��ȣ�ֻҪ��װ����һ������ܸ�����ů�֣���ҷdz����棬��һ�ԣ�������ˣ�ͬѧ�Dz����ԡ�ů���������ڲ��ɷֲ����˼���ĺ��棬��ֽ����ԭ����һЩ����ɫ��ĩ������һ�������ð�̣������֣���ô����Щ��ɫ��ĩ�ɷ��ֻ���ʲô�أ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��̿�����ھ������ڿ��� | |
| B�� | ��C6H10O5��n�����л��߷��ӻ����� | |
| C�� | ��̿��ά����̼Ԫ�ء���Ԫ�غ���Ԫ����ɵ� | |
| D�� | ��̿��ά��C��H��O����Ԫ�ص�������Ϊ6��10��5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
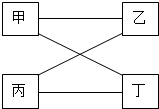 ��ͼ��ʾ�������������˵����ʣ���һ���������¶��ܷ�����ѧ��Ӧ������е�A��B��C��D���������з����������ǣ�������
��ͼ��ʾ�������������˵����ʣ���һ���������¶��ܷ�����ѧ��Ӧ������е�A��B��C��D���������з����������ǣ�������| �� | �� | �� | �� | |
| A | C | O2 | H2SO4 | CuO |
| B | Fe | H2SO4 | CuCl2 | NaOH |
| C | NaOH | CO2 | KOH | CuCl2 |
| D | Na2SO4 | Ba��OH��2 | HCl | Ba��NO3��2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʪ�·���������Ϊˮ�����˶���������ȥ�� | |
| B�� | ����ˮ��ѧ���ʵ���С����ˮ���� | |
| C�� | ˮ��Һ̬�����̬ʱˮ���Ӽ������ | |
| D�� | ˮ�������������ˮ���ӵ����������ɵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ����Һ�� | B�� |  �㵹Һ�� | C�� |  ����ҩƷ | D�� | 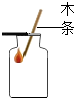 �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȥNaCl��������Na2CO3���ʣ��ȼ�������ϡ���ᣬ�������ᾧ | |
| B�� | �����ȼ���������Ƿ�����Ԫ�أ�����ȼ�ջ����Ϸ����ϸ�����ձ����۲�����ˮ�� | |
| C�� | ʵ��ⶨij��ʯ��̼��Ƶ������������ȼ������ᣬ�ٱȽϷ�Ӧǰ��������仯 | |
| D�� | ʵ�����Ʊ������������������п��Ũ���ᷴӦ�����������徭Ũ���������ռ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com