
”°¶Ō±ČŹµŃé”±ŹĒ»ÆѧѧĻ°ÖŠŠŠÖ®ÓŠŠ§µÄĖ¼Ī¬·½·Ø”£Ä³»ÆѧѧĻ°Š”×éµÄĶ¬Ń§ŌŚŃ§ĶźĻą¹ŲµÄ»ÆѧÖŖŹ¶ŗó£¬×ß½ųŹµŃéŹŅ×öĮĖČēĻĀŹµŃ飬ĒėÄć²ĪÓė²¢»Ų“šĻĀĮŠĪŹĢā”£
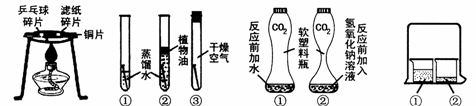
A.Č¼ÉÕµÄĢõ¼ž B. Ģś¶¤ÉśŠāµÄĢõ¼ž C. Ö¤Ć÷CO2ÓėNaOH·“Ó¦ D. ·Ö×ÓŌĖ¶ÆµÄĻÖĻó
£Ø1£©ĶعżŹµŃéA£¬æÉŅŌĖµĆ÷Č¼ÉÕµÄĢõ¼žÖ®Ņ»ŹĒ £¬ŹµŃéÖŠŹ¹ÓĆĶʬ£¬ŹĒĄūÓĆĮĖĶµÄ ŠŌ£ØĢīŅ»ĢõĪļĄķŠŌÖŹ£©
£Ø2£©¶ŌÓŚŹµŃéB£¬Ņ»¶ĪŹ±¼äŗó¹Ū²ģµ½ŹŌ¹Ü¢ŁÖŠµÄĢś¶¤Ć÷ĻŌŠāŹ“£¬ÓÉ“ĖµĆ³ö£ŗĢśÉśŠāµÄÖ÷ŅŖĢõ¼žŹĒĢśÓėĖ®ŗĶ Ö±½Ó½Ó“„”£Óū³żČ„ĢśŠāæÉÓĆ Ļ“µÄ·½·Ø£¬ĢśÖĘĘ·³żŠāŹ± £ØĢī”°ÄÜ”±»ņ”°²»ÄÜ”±£©³¤Ź±¼ä½žŌŚĖįČÜŅŗÖŠ”£
£Ø3£©ŹµŃéCŹĒĄūÓĆĢå»żĻąĶ¬²¢³äĀśCO2µÄČķĖÜĮĻĘ攢µČĮæµÄĖ®£ØĘæ¢Ł£©ŗĶNaOHČÜŅŗ£ØĘæ¢Ś£©½ųŠŠŹµŃ飬øł¾ŻĖÜĮĻĘæ±ä±ńµÄ³Ģ¶ČÖ¤Ć÷CO2 ÓėNaOHČÜŅŗÖŠµÄČÜÖŹČ·Źµ·¢ÉśĮĖ·“Ó¦£¬ÕāŅ»·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©ŹµŃéDµÄÉÕ±¢ŚÖŠ³ŹĻÖµÄĻÖĻóÄÜĖµĆ÷·Ö×ÓŹĒ²»¶ĻŌĖ¶ÆµÄ”£µ±ÉÕ±¢ŁÖŠŅŗĢåŹĒÅØ°±Ė®Ź±ÉÕ±¢ŚÖŠµÄ·ÓĢŖČÜŅŗÓÉĪŽÉ«±äĪŖ É«£»µ±ÉÕ±¢ŁÖŠŅŗĢå»»³ÉÅØŃĪĖį£¬ĒŅÉÕ±¢ŚÖŠŅŗĢå»»³ÉµĪÓŠ·ÓĢŖµÄNaOHČÜŅŗŹ±£¬Ņ»¶ĪŹ±¼äŗó£¬ČÜŅŗŃÕÉ«µÄ±ä»ÆŹĒ ”£ĘäÖŠŃĪĖįÓėNaOH·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £¬ŹōÓŚ ·“Ó¦£ØĢī·“Ó¦ĄąŠĶ£©

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ŹµŃéŹĒ»ÆѧµÄĮ黟£¬ŹĒѧŗĆ»ÆѧµÄÖŲŅŖ»·½Ś£®
ŹµŃéŹĒ»ÆѧµÄĮ黟£¬ŹĒѧŗĆ»ÆѧµÄÖŲŅŖ»·½Ś£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğ½ĖÕŹ”ŃļÖŻŹŠ¾ÅÄź¼¶ÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ŹµŃéŹĒ»ÆѧµÄĮ黟£¬ŹĒѧŗĆ»ÆѧµÄÖŲŅŖ»·½Ś”£
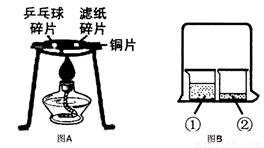
£Ø1£©”¶Č¼ÉÕµÄĢõ¼ž”·ŹµŃéÖŠ£¬ÓĆĆŽ»Ø·Ö±šÕŗ¾Ę¾«ŗĶĖ®£¬·ÅŌŚ¾Ę¾«µĘÉĻ¼ÓČČ£¬øĆŹµŃéµÄÄæµÄĢ½¾æ__________ ____ŹĒČ¼ÉÕµÄĢõ¼žÖ®Ņ»£¬øĆŹµŃéµÄæÉČ»ĪļŹĒÖø__ ________£¬ŹµŃé³É¹¦µÄ¹Ų¼üŹĒæŲÖĘ___ ___²»Č¼ÉÕ”£
£Ø2£©³“²ĖŹ±ÓĶ¹ųÖŠµÄÓĶ²»É÷×Å»š£¬æÉÓĆ¹ųøĒøĒĆš£¬ĘäĆš»šŌĄķĪŖ£ŗ ”£
£Ø3£©”°¶Ō±ČŹµŃé”±ŹĒ»ÆѧѧĻ°ÖŠŠŠÖ®ÓŠŠ§µÄĖ¼Ī¬·½·Ø”£Ä³»ÆѧѧĻ°Š”×éµÄĶ¬Ń§ŌŚŃ§ĶźĻą¹ŲµÄ»ÆѧÖŖŹ¶ŗó£¬×ß½ųŹµŃéŹŅ×öĮĖČēĻĀŹµŃ飬ĒėÄć²ĪÓė²¢»Ų“šĻĀĮŠĪŹĢā”£
¢ŁĶعżĶ¼AŹµŃ飬æÉŅŌĖµĆ÷Č¼ÉÕµÄĢõ¼žÖ®Ņ»ŹĒ £¬ŹµŃéÖŠŹ¹ÓĆĶʬ£¬ŹĒĄūÓĆĮĖĶµÄ ŠŌ___________£ØĢīŅ»ĢõĪļĄķŠŌÖŹ£©”£
¢ŚĶ¼BŹµŃéµÄÉÕ±¢ŚÖŠ³ŹĻÖµÄĻÖĻóÄÜĖµĆ÷·Ö×ÓŹĒ²»¶ĻŌĖ¶ÆµÄ”£µ±ÉÕ±¢ŁÖŠŅŗĢåŹĒÅØ°±Ė®Ź±ÉÕ±¢ŚÖŠµÄ·ÓĢŖČÜŅŗÓÉĪŽÉ«±äĪŖ É«”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŹµŃéŹĒ»ÆѧµÄĮ黟£¬ŹĒѧŗĆ»ÆѧµÄÖŲŅŖ»·½Ś”£
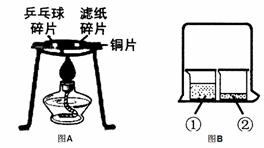
(1)”¶Č¼ÉÕµÄĢõ¼ž”·ŹµŃéÖŠ£¬ÓĆĆŽ»Ø·Ö±šÕŗ¾Ę¾«ŗĶĖ®£¬·ÅŌŚ¾Ę¾«µĘÉĻ¼ÓČČ£¬øĆŹµŃéµÄÄæµÄĢ½¾æ__________ ____ŹĒČ¼ÉÕµÄĢõ¼žÖ®Ņ»£¬øĆŹµŃéµÄæÉČ»ĪļŹĒÖø__ ________£¬ŹµŃé³É¹¦µÄ¹Ų¼üŹĒæŲÖĘ___ ___²»Č¼ÉÕ”£
(2)³“²ĖŹ±ÓĶ¹ųÖŠµÄÓĶ²»É÷×Å»š£¬æÉÓĆ¹ųøĒøĒĆš£¬ĘäĆš»šŌĄķĪŖ£ŗ ”£
(3)”°¶Ō±ČŹµŃé”±ŹĒ»ÆѧѧĻ°ÖŠŠŠÖ®ÓŠŠ§µÄĖ¼Ī¬·½·Ø”£Ä³»ÆѧѧĻ°Š”×éµÄĶ¬Ń§ŌŚŃ§ĶźĻą¹ŲµÄ»ÆѧÖŖŹ¶ŗó£¬×ß½ųŹµŃéŹŅ×öĮĖČēĻĀŹµŃ飬ĒėÄć²ĪÓė²¢»Ų“šĻĀĮŠĪŹĢā”£
¢ŁĶعżĶ¼AŹµŃ飬æÉŅŌĖµĆ÷Č¼ÉÕµÄĢõ¼žÖ®Ņ»ŹĒ £¬ŹµŃéÖŠŹ¹ÓĆĶʬ£¬ŹĒĄūÓĆĮĖĶµÄ ŠŌ£ØĢīŅ»ĢõĪļĄķŠŌÖŹ£©”£
¢ŚĶ¼BŹµŃéµÄÉÕ±¢ŚÖŠ³ŹĻÖµÄĻÖĻóÄÜĖµĆ÷·Ö×ÓŹĒ²»¶ĻŌĖ¶ÆµÄ”£µ±ÉÕ±¢ŁÖŠŅŗĢåŹĒÅØ°±Ė®Ź±ÉÕ±¢ŚÖŠµÄ·ÓĢŖČÜŅŗÓÉĪŽÉ«±äĪŖ É«”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com