
 ĻÖÓŠFeŗĶFe2O3µÄ¹ĢĢå»ģŗĻĪļ£¬¶«¶«Ķ¬Ń§ĪŖĮĖ·ÖĪö»ģŗĻĪļÖŠFeŗĶFe2O3µÄŗ¬Į棬Éč¼ĘĮĖČēĻĀŹµŃé·½°ø£ŗ
ĻÖÓŠFeŗĶFe2O3µÄ¹ĢĢå»ģŗĻĪļ£¬¶«¶«Ķ¬Ń§ĪŖĮĖ·ÖĪö»ģŗĻĪļÖŠFeŗĶFe2O3µÄŗ¬Į棬Éč¼ĘĮĖČēĻĀŹµŃé·½°ø£ŗ
”¾ŹµŃ鏿¾Ż”æŹµŃé¹²¼ĒĀ¼ĮĖĮ½×鏵Ń鏿¾Ż£¬µŚ¢Ł×飬ĘųĢåĶźČ«ĪüŹÕŗó£¬ŹÆ»ŅĖ®ÖŹĮæŌö¼Ó66g£»µŚ¢Ś×飬ĶźČ«·“Ó¦£¬ĄäČ“ŗó³ĘĮæŹ£Óą¹ĢĢåµÄÖŹĮæĪŖWg”£
øł¾ŻŹµŃéÉč¼Ę¼°ÓŠ¹ŲŹż¾Ż½ųŠŠ·ÖĪöÓė¼ĘĖć
£Ø1£©Éś³ÉĘųĢåµÄÖŹĮæŹĒ g”£
£Ø2£©»ģŗĻĪļÖŠFeµ„ÖŹµÄÖŹĮæ·ÖŹżĪŖ¶ąÉŁ£æ£ØŠ“³ö¼ĘĖć¹ż³Ģ£©
£Ø3£©µŚ¢Ś×鏿¾ŻWŹĒ g£ØŠ“³ö¼ĘĖć½į¹ū£©”£
£Ø4£©Ī÷Ī÷Ķ¬Ń§Ģį³ö£¬ÓƱ„ŗĶĒāŃõ»ÆÄĘČÜŅŗČ”“ś³ĪĒåŹÆ»ŅĖ®Ą“ĪüŹÕµŚ¢Ł×é²śÉśµÄĘųĢ劧¹ūøüŗĆ£¬Äć¾õµĆĖūÕāĆ“ĖµµÄĄķÓÉŹĒ”” ”””” ”£
£Ø1£©66£Ø1·Ö£©
£Ø2£©£Ø»Æѧ·½³ĢŹ½2·Ö£¬ĘäĖü¹²4·Ö£©
½ā£ŗ¹ĢĢå»ģŗĻĪļÖŠFe2O3µÄÖŹĮæĪŖX
|
Fe2O3+3CO==== 2Fe +3CO2
 160 132 2·Ö
160 132 2·Ö
X 66g
160:X = 132:66g
½āµĆ x= 80g£Ø1·Ö£©
Feµ„ÖŹµÄÖŹĮæ·ÖŹż£ŗ£Ø100g – 80g£©/100g ”Į100% = 20%£Ø1·Ö£©
“š£ŗ»ģŗĻĪļÖŠĢśµ„ÖŹµÄÖŹĮæ·ÖŹżŹĒ20%”£
£Ø3£©76£Ø2·Ö£©
£Ø4£©ŅņĪŖĒāŃõ»ÆøĘĪ¢ČÜÓŚĖ®£¬¶ųĒŅĻąĶ¬ÖŹĮæµÄ±„ŗĶµÄĒāŃõ»ÆÄĘČÜŅŗ±ČŹÆ»ŅĖ®ÄÜĪüŹÕøü¶ąµÄ¶žŃõ»ÆĢ¼”££Ø2·Ö£©


 æĘѧŹµŃé»ī¶Æ²įĻµĮŠ“š°ø
æĘѧŹµŃé»ī¶Æ²įĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2013½ģ¹ć¶«Ź”·šÉ½ŹŠģų³ĒĒų³õČżµŚŅ»Ń§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗ¼ĘĖćĢā
ĻÖÓŠFeŗĶFe2O3µÄ¹ĢĢå»ģŗĻĪļ£¬ĪŖĮĖ·ÖĪö»ģŗĻĪļÖŠFeŗĶFe2O3µÄŗ¬Į棬Éč¼ĘĮĖČēĻĀŹµŃé·½°ø£ŗ
”¾ŹµŃ鏿¾Ż”æŹµŃé¹²¼ĒĀ¼ĮĖĮ½×鏵Ń鏿¾Ż£¬µŚ¢Ł×飬ĘųĢåĶźČ«ĪüŹÕŗó£¬ŹÆ»ŅĖ®ČÜŅŗÖŹĮæŌö¼Ó66g£»µŚ¢Ś×飬ĶźČ«·“Ó¦£¬ĄäČ“ŗó³ĘĮæŹ£Óą¹ĢĢåµÄÖŹĮæĪŖWg”£
Fe2O3ŗĶCO·“Ó¦·½³ĢŹ½ŹĒ£ŗFe2O3 + 3CO 2Fe + 3CO2
2Fe + 3CO2
øł¾ŻŹµŃéÉč¼Ę¼°ÓŠ¹ŲŹż¾Ż½ųŠŠ·ÖĪöÓė¼ĘĖć
£Ø1£©Éś³ÉĘųĢåµÄÖŹĮæŹĒ g”£
£Ø2£©»ģŗĻĪļÖŠFeµ„ÖŹµÄÖŹĮæ·ÖŹżĪŖ¶ąÉŁ£æ£ØŠ“³ö¼ĘĖć¹ż³Ģ£©
£Ø3£©µŚ¢Ś×鏿¾ŻWŹĒ g£ØŠ“³ö¼ĘĖć½į¹ū£©”£
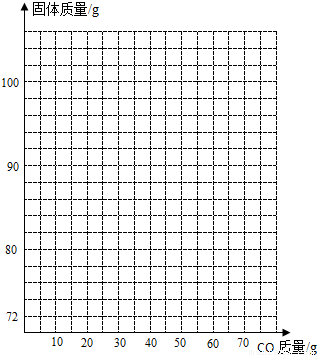
£Ø4£©ČōŌŚ100g FeŗĶFe2O3µÄ¹ĢĢå»ģŗĻĪļÖŠ³ÖŠų²»¶ĻµÄĶØČėCO£¬ĒėÄć»³ö¹ĢĢåÖŹĮæÓėĶØČėCOÖŹĮæµÄ¹ŲĻµĶ¼£ØŌŚ“šĢāæصÄ×ų±źÖŠ×÷Ķ¼£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ¹ć¶«Ź”½ŅŃōŹŠ»ŻĄ“ĻŲæūĢ¶ÖŠŃ§¾ÅÄź¼¶£ØĻĀ£©ĘŚÄ©»ÆŃ§Ä£ÄāŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ¹ć¶«Ź”·šÉ½ŹŠģų³ĒĒų¾ÅÄź¼¶£ØÉĻ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
 2Fe+3CO2
2Fe+3CO2²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com