
 CO2+2H2O��˵�����۹ܵ����к��м��飬Ҫ�������ɵĶ�����̼����ʹ�ó���ʯ��ˮ������ʯ��ˮ����Ǽ���֤�������˶�����̼��
CO2+2H2O��˵�����۹ܵ����к��м��飬Ҫ�������ɵĶ�����̼����ʹ�ó���ʯ��ˮ������ʯ��ˮ����Ǽ���֤�������˶�����̼�� CO2+2H2O�������ļ���������ȫȼ������ˮ�������̼��������=��2×18����44=9��11��������۹ܵ�������CO��ȼ���������ж�����̼������������Ӧ��
CO2+2H2O�������ļ���������ȫȼ������ˮ�������̼��������=��2×18����44=9��11��������۹ܵ�������CO��ȼ���������ж�����̼������������Ӧ��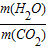 ��9��11��
��9��11�� CO2+2H2O������ʯ��ˮ
CO2+2H2O������ʯ��ˮ


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
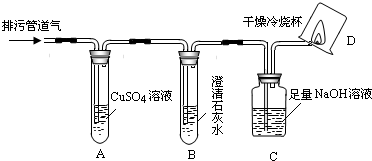
| m(H2O) | m(CO2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

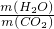 ��______����ԭ��Ʒ���к���CO������ԭ��Ʒ���оͲ���CO�����밴��˼·���������������գ���
��______����ԭ��Ʒ���к���CO������ԭ��Ʒ���оͲ���CO�����밴��˼·���������������գ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0119 ��ĩ�� ���ͣ�ʵ����

 <____________����ԭ��Ʒ���к���CO������ԭ��Ʒ���оͲ���CO���밴��˼·���������������ա�
<____________����ԭ��Ʒ���к���CO������ԭ��Ʒ���оͲ���CO���밴��˼·���������������ա� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
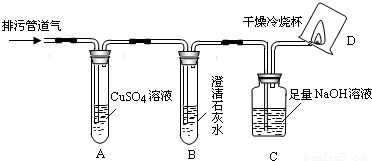
�������۹ܵ��г���ۼ��ж���������������¹ʡ�ijѧϰС����֪���������۹ܵ����ijɷ���ʲô������ͨ���������ϵ�֪��
I���������۹ܵ��п��ܺ��нϴ�����CO��CO2��H2S��CH4�����塣
��H2S�����о綾������CuSO4��Һ��Ӧ���ɺ�ɫ������
��.CO2�ᱻNaOH��Һ���գ� CH4��CO������CuSO4��Һ��ʯ��ˮ�Լ�NaOH��Һ��Ӧ��
��С���������ͼ��ʾ��װ�ò�����̽��(�г�������ʡ��)��
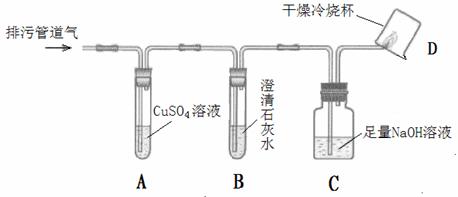
��ش��������⣺
��1��װ��A�г��ֺ�ɫ���ǣ�˵�����۹ܵ����к��� ���ѧʽ����
��2��װ��B�г��ְ�ɫ���ǣ�˵�����۹ܵ����к��� ���ѧʽ�����˷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��װ��D�пɼ����棬������ձ��ڱ���ˮ�����֣�˵�����۹ܵ����к��� ���ѧʽ�����˷�Ӧ�Ļ�ѧ����ʽΪ ��
![]() ��Ҫ��֤������ȼ�պ����һ�ֲ���ɽ��еIJ����ǣ�Ѹ�ٰ��ձ������������ձ���ע�� ����
��Ҫ��֤������ȼ�պ����һ�ֲ���ɽ��еIJ����ǣ�Ѹ�ٰ��ձ������������ձ���ע�� ����
��4����ͬѧ��������ȼ�ղ�����ˮ�Ͷ�����̼�����������㣻�� < ����ԭ��Ʒ���к���CO������ԭ��Ʒ���оͲ���CO���밴��˼·���������������ա�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com