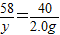С��ͬѧ�Ի�ѧ��������Ȥ��ϲ��������ʵ�飮��һ����������ͬѧ��þ������һЩ�о���
����������ɰֽ��ĥ������Һ�ɫ�����ʣ��������ˮ�У�δ���������Ե������þƾ��Ƽ��Ⱥ۲쵽�����д������ݣ�����������������������Һ�еμ���ɫ��̪��Һ�����ַ�̪��ɺ�ɫ��˵����
OH-
OH-
��������ʵ�������ѧ��Ӧ�ķ�����������
�¶�
�¶�
�йأ��������ʵ�
��Ӧ�ٶ�
��Ӧ�ٶ�
���¶ȵ����߶�����
����С���Ա��ʼ�о�þ������ĻҺ�ɫ���ʣ�С����������������裺
����1��������MgO������2��������Mg��OH��
2������3þ���ܻ���ͭһ�����ɼ�ʽ̼��þ��
������ͬ�������������ļ���1��2����Ϊ������ѧ��֪ʶMgO��Mg��OH��
2����
��
��
ɫ���壮���ڼ���3��������������·���
| ʵ�鷽�� |
ʵ������ |
ʵ����� |
|
|
֤����CO32- |
| ȡ�����Թܣ����� |
|
֤������Ԫ�� |
��III��������ȡþ������Һ�ɫ��Ʒʱ�е��Ƚ����ѣ����Խ����淢�ڵ�þ��ȫ��ĥ�ɷ�ĩ����������Ӳ���Թ��м��ȣ�һ��ʱ���þ��ͻȻ���ֺ���ȼ����������Ӧֹͣ����������ɫ����Aճ���Թܱ��ϣ����ɫ����A�л���ʲô�����Dz�����ɸѡ�������м�ֵ����Ϣ��
��1��2Mg+CO
2=2MgO+C�� ��2��C+2H
2SO
4��Ũ��=CO
2��+SO
2��+H
2O����3��SO
2+Ca��OH��
2=CaSO
3�����ף�+H
2O��
�Է���A����Ϊ
C
C
������������Ϣ�����������������ʵ�飬�����������ʵ�鱨�棺
| ʵ�鷽�� |
ʵ������ |
ʵ����� |
| ȡ��Ӧ���Թ�����Ʒ���ȵμӹ����� ϡ���� ϡ���� |
���岿���ܽ� |
|
| �� ���� ���� ����һ�����ʵ������� |
��ɫ����ȫ���ܽ� |
|
������Ϊ��֤̼��Ũ���ᷴӦ�����ֲ��Ӧ������ͨ�����������Լ�������ÿ������ȫ������
�����
��ˮ����ͭ
��ˮ����ͭ
��
���������Һ
���������Һ
��
����ʯ��ˮ
����ʯ��ˮ
��
������������
��ɫ�������
��ɫ�������
��
�Ϻ�ɫ��ȥ
�Ϻ�ɫ��ȥ
��
����ʯ��ˮ�����
����ʯ��ˮ�����
��V������ȤС���ͬѧ��һ���ֲ��θҩ��ʽ̼��þ�ж��ֲ�ͬ����ɣ���Mg
2��OH��
2CO
3��Mg
4��OH��
2��CO
3��
3��Mg
5��OH��
2��CO
3��
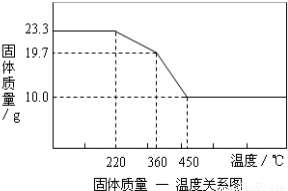
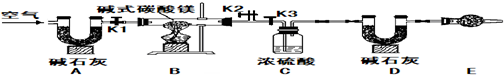
4�ȣ�С��ͬѧΪȷ����ʽ̼��þ����ɣ������ͼ��ʾ��ʵ�飺
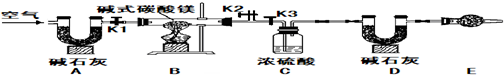
ʵ�鲽�裨1����ͼ��װ�ú����Ƚ��еIJ�����
���װ�õ�������
���װ�õ�������
��E���������ʢ�ŵ�ҩƷ��
��ʯ��
��ʯ��
����������
��ֹ�����е�ˮ��������װ��
��ֹ�����е�ˮ��������װ��
��
ʵ�鲽�裨2����ȡ��Ʒ31g�����������Ӳ�ʲ������У�����װŨ�����ϴ��ƿC������Ϊ87.6g��װ��ʯ�ҵ�U��D������Ϊ74.7g��
ʵ�鲽�裨3������
K1K2
K1K2
���ر�
K3
K3
������������������ӣ�
ʵ�鲽�裨4���رջ���
K1K2
K1K2
����
K3
K3
����ȼ�ƾ��Ƽ��������ٲ�������Ϊֹ��
ʵ�鲽�裨5������K1������������������ӣ�Ȼ�����װ�ã��Ƶ�ϴ��ƿC������Ϊ89.4g��U��D������Ϊ87.9g����ü�ʽ̼��þ�Ļ�ѧʽΪ
Mg4��OH��2CO3
Mg4��OH��2CO3
��
������С����Ϊ�仯ѧʽ����Mgx��OH��
2��CO
3��y��ʾ�����ݻ��ϼ۹��ɣ�x��y�Ĺ�ϵ��
x=y+1
x=y+1
��д�������ʷֽ�ķ���ʽ��
Mgx��OH��
2��CO
3��
yxMgO+H
2O+yCO
2��
Mgx��OH��
2��CO
3��
yxMgO+H
2O+yCO
2��
С�������������������װ����ԭ�������壩
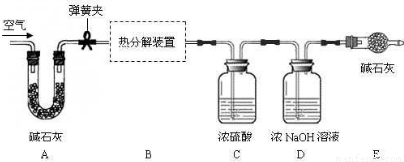
����I��ֻ�ⶨ�ֽ�����ɵ�ˮ�����ӷ�����A��C��B����C��ʢ�ŵ�ҩƷ��
Ũ����
Ũ����
��B��ʢ�ŵ�ҩƷ��
�Ȼ��ƹ���
�Ȼ��ƹ���
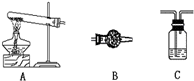
����II��ֻ�ⶨ�ֽ����ɵ�CO
2�����������ӷ�����A��C��B��C����B�е�ҩƷ��
��ʯ��
��ʯ��
��������ӵ�C�е�ҩƷ��
����������Һ
����������Һ
������Ƴ�����ʵ�鷽��
���ȼ�ʽ̼��þ������Ӧǰ���������
���ȼ�ʽ̼��þ������Ӧǰ���������
��
��ȡ��Ʒ7.88�ˣ�����I�������ˮ0.36g������II�������CO
2 3.52g������ѡ��ʵ�����ݣ�ͨ������ó���ʽ̼��þ�Ļ�ѧʽ��