��2013?̫ԭ����ѧС��ͬѧΪ����ij�����ų�����ɫ��ˮ�ɷ֣����Dz��ĸù������й����Ϻ��Ʋ����ˮ�п��ܺ���HCl��Na
2SO
4��Na
2CO
3�е�һ�ֻ������ʣ�ͬѧ��ȡ��ˮ����������̽��������֪Na
2SO
4��Һ�����ԣ�
��1������ˮ��pH��ȡһ��pH��ֽ�����ڲ���Ƭ��
�ò�����պȡ��ˮ��Ʒ������pH��ֽ�ϣ������ɫ���Ƚ�
�ò�����պȡ��ˮ��Ʒ������pH��ֽ�ϣ������ɫ���Ƚ�
������ΪPh=2���ɴ˿�֪����ˮ��һ����
HCl
HCl
��������Na
2SO
4��
��2������Na
2SO
4�Ƿ���ڣ���ͬѧ��һ֧�Թ���ȡ������ˮ�����Թ��еμ�������
BaCl2
BaCl2
��Һ���������˰�ɫ��������Ӧ�Ļ�ѧ����ʽΪ
Na2SO4+BaCl2�TBaSO4��+2NaCl
Na2SO4+BaCl2�TBaSO4��+2NaCl
���������ɣ���֤����ˮ����Na2SO4���ڣ���ͬѧ����������Ҳ�õ���ͬ���Ľ��ۣ�����ʵ�������������
���Թ���ȡ������ˮ��Ʒ���ɣ��а�ɫ��������
���Թ���ȡ������ˮ��Ʒ���ɣ��а�ɫ��������
��
ͨ��̽����ͬѧ��ȷ������ˮ�ijɷ֣�
Ϊʹ�ŷŵ���ˮ�в��������������ˮ�мӹ�����
ʯ��ʯ������м��
ʯ��ʯ������м��
���������ɣ���



 �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�

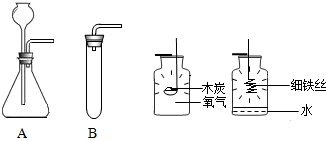
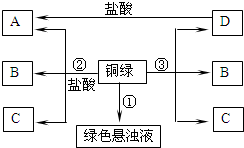 ��2013?�����ģ�⣩Сͮ��һ���۾�����һ��ʱ�����ͭ�ʾ����ϳ�����һ����ɫ���ʣ����뽫����������������ϵ�֪��ͭ��һ�������»���ʴ����һ����ɫ���ʣ�����Ҫ�ɷ��Ǽ�ʽ̼��ͭ[�׳�ͭ�̣���ѧʽΪCu2��OH��2CO3]�����������Ȳ��ȶ��ֽ⣮������Ԫ���غ�������ó�ͭ������ͭ������е�������������̼��
��2013?�����ģ�⣩Сͮ��һ���۾�����һ��ʱ�����ͭ�ʾ����ϳ�����һ����ɫ���ʣ����뽫����������������ϵ�֪��ͭ��һ�������»���ʴ����һ����ɫ���ʣ�����Ҫ�ɷ��Ǽ�ʽ̼��ͭ[�׳�ͭ�̣���ѧʽΪCu2��OH��2CO3]�����������Ȳ��ȶ��ֽ⣮������Ԫ���غ�������ó�ͭ������ͭ������е�������������̼��