
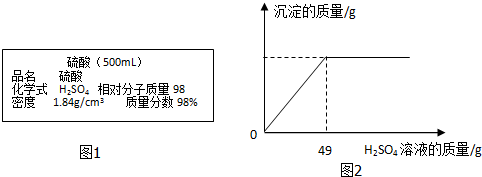
���� ��1������m=��V���ɱ�ǩ����ע��Ũ�����������ܶȼ����ƿŨ��������������ݼ�ˮ����ǰ����Һ������������������䣬�������ˮϡ��ʱ����98%��Ũ������ˮ�������ȣ�
��2���Ȼ��ƺ��Ȼ����Ļ����Һ��ֻ���Ȼ����������ᷴӦ�������ᱵ�������⻯�⣬�ɷ�Ӧ������ͼ�ɵ�֪������ϡ����49gʱ��Ӧǡ����ɣ�����������������������ݷ�Ӧ�Ļ�ѧ����ʽ������μӷ�Ӧ���Ȼ������������Ȼ��Ƶ�����=32.8g��������-�Ȼ�����������
��3�����ݷ�Ӧ�Ļ�ѧ����ʽ���ɲμӷ�Ӧ��������������������⻯���������
��� �⣺��1��������Һ������=500mL��1.84g/cm3=920g��
��ϡ�ͳ�20%��ϡ����ʱ����98%��Ũ������ˮ��������a��b
a g��98%=��a g+b g����20% ���a��b=10��39
�ʴ�Ϊ��920��10��39��
��2����3�������ϡ����պ�ʹ���������ʱ���μӵķ�Ӧ�������������49g��20%=9.8g
��32.8g�����к���BaCl2������Ϊx�������ɵ�HCl������Ϊy��
BaCl2+H2SO4=BaSO4��+2HCl
208 98 73
x 9.8g y
$\frac{208}{x}=\frac{98}{9.8g}=\frac{73}{y}$
x=20.8g y=7.3g
��2���Ȼ�������Ϊ��32.8g-20.8g=12g
��3��HCl��������=$\frac{7.3g}{200g}��$100%=3.65%
�𰸣�
��1��920 10��39
��2��32.8g�����������Ȼ���Ϊ20.8g���Ȼ��Ƶ�����Ϊ12g��
��3����Һ������HCl��������Ϊ3.65%��
���� ���ò������������������ϡ����������Ĺ�ϵͼ���۵㣬������ʱ����ϡ����ǡ����ȫ��Ӧ���ҳ��μӷ�Ӧ������������ǽ���������㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ơ�� | B�� | ���� | C�� | ������̼ | D�� | ��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ƾ���ȼ�� | B�� | ͭ�ܵ��� | ||
| C�� | �������������� | D�� | ������̼��ʹ����ʯ��ˮ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ԭ�������������Բ���Ԫ�ص����ԭ������ | |
| B�� | ��������ε��ܽ��Ա��������жϵó�����ε��ܽ����ֵ��С | |
| C�� | ���ܽ������ͼ����ѡ�����Һ�л�þ���ķ��� | |
| D�� | ���ݿ��������ձ�����֪���������������Ҫ��Ⱦ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��ȡҺ����� | B�� |  ���� | C�� |  �㵹Һ�� | D�� |  ��ȡ������̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com