

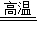 Na2S+1CO2��+1CO�����÷�Ӧ����ͬ����4���õķ�Ӧ����ʽΪ��3Na2SO4+8C
Na2S+1CO2��+1CO�����÷�Ӧ����ͬ����4���õķ�Ӧ����ʽΪ��3Na2SO4+8C 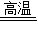 3Na2S+4CO2��+4CO����
3Na2S+4CO2��+4CO����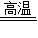 3Na2S+4CO2��+4CO��
3Na2S+4CO2��+4CO��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
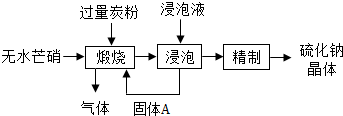 ��2012?��������ģ����������Ҫ������Ʒ����ҵ�ϲ�����ˮâ����Na2SO4��-̿�ۻ�ԭ���������ƣ�Na2S����������ʾ��ͼ��ͼ��
��2012?��������ģ����������Ҫ������Ʒ����ҵ�ϲ�����ˮâ����Na2SO4��-̿�ۻ�ԭ���������ƣ�Na2S����������ʾ��ͼ��ͼ��
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�����й�����2012����꼶�п���ģ��ѧ���� ���ͣ�058
��������Ҫ������Ʒ����ҵ�ϲ�����ˮâ��(Na2SO4)��̿�ۻ�ԭ����������(Na2S)��������ʾ��ͼ���ϣ�
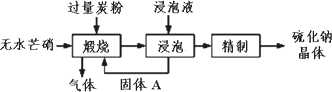
(1)���������У���ù���A�IJ����ǹ��ˣ�����ֽ����ͨ©���ڱڲ����������µĺ����________������A�Ļ�ѧʽΪ________����������Ԫ�صĻ��ϼ�Ϊ________�ۣ�
(2)������̿�۵����ó����ṩ��ԭ���⣬���ܾ���________����
(3)�������ա����������Ƿ��Ӹ�����Ϊ1��1������̼������������ա�ʱ�������ܵĻ�ѧ��Ӧ����ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��������Ҫ������Ʒ����ҵ�ϲ�����ˮâ����Na2SO4��-̿�ۻ�ԭ���������ƣ�Na2S����������ʾ��ͼ��ͼ��
��������Ҫ������Ʒ����ҵ�ϲ�����ˮâ����Na2SO4��-̿�ۻ�ԭ���������ƣ�Na2S����������ʾ��ͼ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�꽭��ʡ�����й������п���ѧ��ģ�Ծ��������棩 ���ͣ������
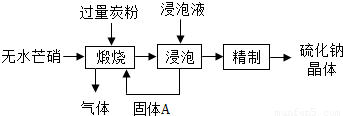
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com