


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���������ͼ��ʾ�ش��������⣺
���������ͼ��ʾ�ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�챱���з�ɽ�����꼶��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��8�֣�����������������벻��������
��1����ͭ�Ƶ�����Ҫ������ͭ�������õ� �ԡ�
��2�����dz��á�ͭǽ���ڡ�����������ļ�̡�������һ��������Ҳ�ܷ������ַ�Ӧ��
����˿��������ȼ�գ���Ӧ�Ļ�ѧ����ʽ��________________________________��
��3������Ʒ��ʴ��ʵ���������������е� �����˻�ѧ��Ӧ����ֹ����������
ʴ��һ�ַ����� ________________________ ��
��4��������ͼ��ʾ�ش�������һ���еĽ����� ____ ��������ϡ���ᷴӦ�Ļ�ѧ��
��ʽΪ_______________________________________��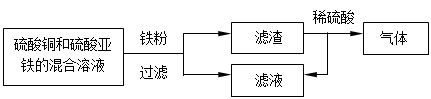
��5����ijϡ�����Ϊ����������ݣ����������ձ��У��ֱ�����������п�������ֽ�
������Ӧ�������û��ʣ��,���������������淴Ӧʱ��仯������ͼ������˵��
��ȷ����___________��������ĸ��ţ�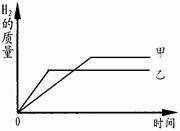
| A��������������п |
| B�������������������� |
| C���μӷ�Ӧ��п������С���������� |
| D����ַ�Ӧ��ϡ����һ������ʣ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�걱���з�ɽ�����꼶��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��8�֣�����������������벻��������
��1����ͭ�Ƶ�����Ҫ������ͭ�������õ� �ԡ�
��2�����dz��á�ͭǽ���ڡ�����������ļ�̡�������һ��������Ҳ�ܷ������ַ�Ӧ��
����˿��������ȼ�գ���Ӧ�Ļ�ѧ����ʽ��________________________________��
��3������Ʒ��ʴ��ʵ���������������е� �����˻�ѧ��Ӧ����ֹ����������
ʴ��һ�ַ����� ________________________ ��
��4��������ͼ��ʾ�ش�������һ���еĽ����� ____ ��������ϡ���ᷴӦ�Ļ�ѧ��
��ʽΪ_______________________________________��

��5����ijϡ�����Ϊ����������ݣ����������ձ��У��ֱ�����������п�������ֽ�
������Ӧ�������û��ʣ��,���������������淴Ӧʱ��仯������ͼ������˵��
��ȷ����___________��������ĸ��ţ�

A��������������п
B��������������������
C���μӷ�Ӧ��п������С����������
D����ַ�Ӧ��ϡ����һ������ʣ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ģ���� ���ͣ������
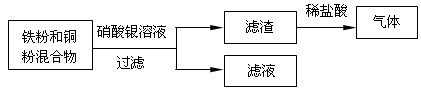
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com