
��4��)���������ũҵ�������벻��ˮ����ͼ������ˮ������ˮ�Ĺ���ʾ��ͼ��
�����ͼʾ�ش��������⣺

��1������ˮ����������ˮʱ��ʹ�õľ�ˮ������___________������Ŷ�ѡ����
A������ B������ C����� D������ E������
(2)��һ���������̶���Խϸߵ��� ___________������ѡ��
A.���� B������ C������ D������
��3��ȡˮ���������������������������_______________��
��4������ˮ����ClO2������Ư��[��Ч�ɷ�ΪCa(ClO)2]����������ˮ��������ҵ���Ʊ�Ư�۵Ļ�ѧ����ʽΪ��2Cl2��2Ca(OH)2 = X��Ca(ClO)2��2H2O,
X�Ļ�ѧʽΪ___________ ��
��1�� A B E (2) D (3) ���� ��4��CaCl2
�������������1������ˮ����������ˮʱ��ʹ�õľ�ˮ�����г��������ˡ�������
2��������ˮ�����̶���Խϸߵķ�����
3������������������������С����������ã�
4���ڻ�ѧ����ʽ2Cl2��2Ca(OH)2 = X��Ca(ClO)2��2H2O�У�X�Ļ�ѧʽΪ��CaCl2
���㣺ˮ�ľ���������ѧ����ʽ�������غ㶨�ɡ�
��������ѧ��Ӧǰ��ԭ�ӵ����ࡢ���������������ı䣬�������ԭ���������ƶϳ���ѧ����ʽ��ij���ʵĻ�ѧʽ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����б�ҵ��ѧ���ԣ��Ĵ��Թ�������ѧ���������� ���ͣ������
(4��)���������ũҵ�������벻��ˮ����ͼ������ˮ������ˮ�Ĺ���ʾ��ͼ��
�����ͼʾ�ش��������⣺
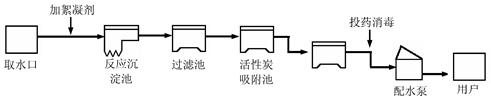
��1������ˮ����������ˮʱ��ʹ�õľ�ˮ������___________������ţ���
| A������ | B������ | C����� | D������ E������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ���꼶11���¿���ѧ�Ծ��������棩 ���ͣ�����⡣
��4��)���������ũҵ�������벻��ˮ����ͼ������ˮ������ˮ�Ĺ���ʾ��ͼ��
�����ͼʾ�ش��������⣺

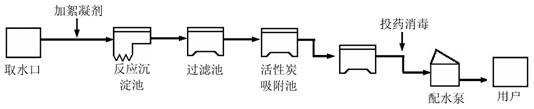
��1������ˮ����������ˮʱ��ʹ�õľ�ˮ������___________������Ŷ�ѡ����
A������ B������ C����� D������ E������
(2)��һ���������̶���Խϸߵ��� ___________������ѡ��
A.���� B������ C������ D������
��3��ȡˮ���������������������������_______________��
��4������ˮ����ClO2������Ư��[��Ч�ɷ�ΪCa(ClO)2]����������ˮ��������ҵ���Ʊ�Ư�۵Ļ�ѧ����ʽΪ��2Cl2��2Ca(OH)2 = X��Ca(ClO)2��2H2O,
X�Ļ�ѧʽΪ___________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����б�ҵ��ѧ���ԣ��Ĵ��Թ�������ѧ�������棩 ���ͣ������
(4��)���������ũҵ�������벻��ˮ����ͼ������ˮ������ˮ�Ĺ���ʾ��ͼ��
�����ͼʾ�ش��������⣺

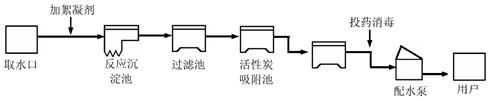
��1������ˮ����������ˮʱ��ʹ�õľ�ˮ������___________������ţ���
A������ B������ C����� D������ E������
��2��ȡˮ���������������������������________________________________________��
��3������ˮ����ClO2������Ư��2����������ˮ��������ҵ���Ʊ�Ư�۵Ļ�ѧ����ʽΪ��2Cl2��2Ca(OH)2=X��Ca(ClO)2��2H2O, X�Ļ�ѧʽΪ ��
��4����Լ��ˮ����������ٳ�һ�ֽ�ˮ�Ĵ�ʩ________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com