��ѧ��ת��Ϊ���ܣ���������������������Ҫ�����ã�

������ȼ�ϵ���Ƿ�����ɫ��ѧ��������͵�أ�
ͼһ������ȼ�ϵ�ص�ʾ��ͼ������ʱH
2��O
2��Ӧ����ˮ��ͬʱ�ṩ���ܣ���Һ��pH
��С
��С
����������С���������䡱����
��ijѧУ�о�С��Էϸɵ���ڵĺ�ɫ���ʽ����о���
�����ϵ�֪��
�ٷϸɵ���ڿ��ܺ���C��MnO
2��NH
4Cl��ZnCl
2�ȣ�
��NH
4Cl�����̬���ʵ����ʣ�
����ZnCl
2��Һ����ε���ϡ��ˮ��������Zn��OH��
2��ɫ������Ȼ������ܽ⣬���ɿ����Ե�[Zn��NH
3��
4]Cl
2��
�ݴˣ�����ȤС��ͬѧ����������ʵ��̽����
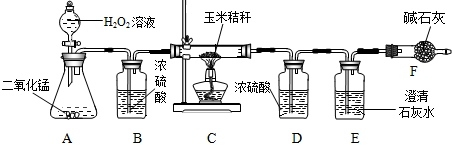
��1������MnO
2�Ĵ��ڣ���ȤС��ͬѧ�������ͼ����ʾ��ʵ�飺
�����ڵ�������
����
����
�����������������
����
����
��
���������պ�ɫ����ʱ��������Ҫ������
�ƾ���
�ƾ���
������������������Ǽܣ������չ����л����һ����ʹ����ʯ��ˮ����ǵ����壬��������
CO2
CO2
��д��ѧʽ����
�����ܵ��Թ��в�����������ʹ�����ǵ�ľ����ȼ����������
O2
O2
��д��ѧʽ����
�ɴ˵õ����ۣ������г��˺�
C
C
�⣬������MnO
2��
��2������1����Һ�����ʵ���Ҫ�ɷ�ΪNH
4Cl��
������ȡ������Һ����ʢ��NaOH������Թܣ��������ȣ��д̼�����ζ�������������ʪ���ɫʯ����ֽ�����Թܿڣ���ֽ������
ʵ����ۣ�
��Һ�����ʵ���Ҫ�ɷ�ΪNH4Cl
��Һ�����ʵ���Ҫ�ɷ�ΪNH4Cl
��
��3������2����Һ�����ʵ���Ҫ�ɷ�ΪZnCl
2��������ʢ��������Һ���Թ�����μ���
ϡ��ˮ
ϡ��ˮ
���۲쵽
���а�ɫ�������ɣ�Ȼ��������ܽ�
���а�ɫ�������ɣ�Ȼ��������ܽ�
��
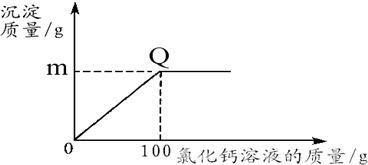
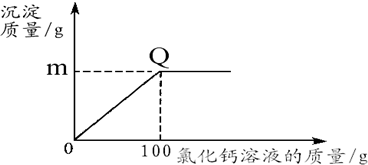
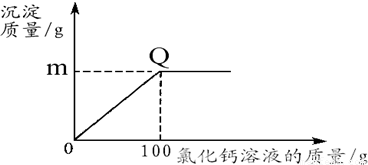
ʵ����ۣ���Һ�����ʵ���Ҫ�ɷ�ΪZnCl
2��4��ZnCl
2��NH
4Cl�����Һ�к�������CuCl
2������ٶԵ�صĸ�ʴ�������ڻ����Һ�Ļ���ʹ�ã�����ȥ���е�Cu
2+�����ѡ�������Լ��е�
B
B
����д��ĸ��ţ���
A��NaOH B��Zn C��Na
2CO
3 D��Fe��