
��6�֣���ش������йء��߽���̼�������⣺
1.�������ڡ����ɻ������������� ������ţ���
�ٷ���ҵ�� �ڿ�Ȫˮƿ ��ù����� ���ƾ���Ͱ
2.���з��š���̼������������ȼ���� ��
��ú ������ ����Ȼ�� ������
3.̼������Һ��ϡ������Է�Ӧ���ɶ�����̼��Ϊ�ⶨijNa2CO3��Һ�����������������ֳ�ȡ10g̼������Һ�����ձ��в�����ϡ���ᣬ������������������ϡ�����������ϵ��ͼ��ʾ���Լ��㣺
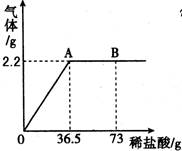
�ٶ�ͼ��֪������������̼������Ϊ ��
�ڸ���Һ��̼���Ƶ�����Ϊ ����������Ϊ ��
��A��ʱ���ձ�����Һ�����ʵĻ�ѧʽ ��
1.��
2.��
3.��2.2g ��5.3g��53% ��NaCl ����1�֣�
��������(1) ù����������������ˣ������ڲ��ɻ���������2������ȼ�ղ�����ˮ��������������������Դ����3����ͼ��֪������������̼������Ϊ2.2g���ݴ˿��Ը��ݻ�ѧ����ʽ���㣬����Һ��̼���Ƶ�����ΪX��
2HCl+ Na2CO3== 2NaCl+H2O+CO2��
106 44
X 2.2g
106/44=X/2.2g�����X=5.3g������������Ϊ5.3g/10g*100%=5.3%
A��ʱ��ǡ����ȫ��Ӧ�������ձ�����Һ������ֻ���Ȼ��ƣ��仯ѧʽΪNaCl


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?������һģ����ش������йء��߽���̼�������⣺
��2012?������һģ����ش������йء��߽���̼�������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ�����л������п�һģ��ѧ�Ծ� ���������� ���ͣ������
��6�֣���ش������йء��߽���̼�������⣺
��С��1���������ڡ����ɻ������������� ������ţ���
�ٷ���ҵ�� �ڿ�Ȫˮƿ ��ù����� ���ƾ���Ͱ
��С��2�����з��š���̼������������ȼ���� ��
��ú ������ ����Ȼ�� ������
��С��3��̼������Һ��ϡ������Է�Ӧ���ɶ�����̼��Ϊ�ⶨijNa2CO3��Һ�����������������ֳ�ȡ10g̼������Һ�����ձ��в�����ϡ���ᣬ������������������ϡ�����������ϵ��ͼ��ʾ���Լ��㣺
�ٶ�ͼ��֪������������̼������Ϊ ��
�ڸ���Һ��̼���Ƶ�����Ϊ ����������Ϊ ��
��A��ʱ���ձ�����Һ�����ʵĻ�ѧʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��㶫ʡ��ݸ���п���ѧ�Ծ��������棩 ���ͣ������
 C2H4+ ��
C2H4+ ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com