
 ����μӵĻ�ѧʵ�鿼�����Ŀ�ǡ���εļ��顱���Իش���������
����μӵĻ�ѧʵ�鿼�����Ŀ�ǡ���εļ��顱���Իش���������
| ||
| ||
| ��� | �۷�ԭ�� |
| 1 | �Թ���Һ��̫�� �Թ���Һ��̫�� |
| 2 | ����ʱ��Ĵָ�����ԹܼеĶ̱� ����ʱ��Ĵָ�����ԹܼеĶ̱� |
| ||
| ||


 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������г��л�ѧʵ�鿼��������ǡ�ʵ������ȡ������̼���塱����ش�����������⣮
�������г��л�ѧʵ�鿼��������ǡ�ʵ������ȡ������̼���塱����ش�����������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����μӵĻ�ѧʵ�鿼�����Ŀ�ǡ���εļ��顱���Իش���������
����μӵĻ�ѧʵ�鿼�����Ŀ�ǡ���εļ��顱���Իش���������| ��� | �۷�ԭ�� |
| 1 | ______ |
| 2 | ______ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�п����� ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������г��л�ѧʵ�鿼��������ǡ�ʵ������ȡ������̼���塱����ش�����������⡣
(1)�����й��������������������У�������ǣ� (�����)��
 A������������Լ����֮һ�����Թܿ�
A������������Լ����֮һ�����Թܿ�
B���齺���벣����������ǰ��ˮ��ʪ�ܿ�
C���齺��һ����������������
D���������ܿ�һ�������齺��һ��
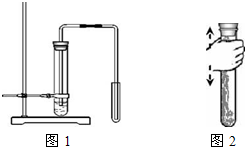
(2)��ͼ1��ij����Ƶ��Ʊ�������̼��ʾ��ͼ��
������ ������
��д��ʵ�����Ʊ�������̼����Ļ�ѧ����ʽ
��
�ۡ�ʵ���Ǽ��黯ѧԭ������߷�ͥ���������й�ʵ����
�ռ�������̼�����������У�������ʵ����ʵ���ǣ�
(�����)�� ͼ1
A����ΪCO2������ˮ��һ�㲻����ˮ���ռ�CO2
B����ΪCO2�ܽ��Բ���ǿ����ˮ�� Ӧ���ɵ�̼���ֺܲ��ȶ���������ˮ���ռ�CO2
Ӧ���ɵ�̼���ֺܲ��ȶ���������ˮ���ռ�CO2
C����ˮ���ռ�CO2ʱ����۲쵹�õ��Թ���Һ���½��������ݴ��Թܿ��ݳ�
D��ͨ��״���£�CO2�ܶȴ��ڿ�����ֻ���������ſ������ռ�CO2
E����ΪCO2����ɫ���壬�ſ������ռ�CO2ʱ������ȼ�ŵ�ľ����������
 (3)��ͼ2��ʾ������CO2���Թ��ڵ���Լռ�Թ��ݻ�����֮һ�ij���ʯ��ˮ��
(3)��ͼ2��ʾ������CO2���Թ��ڵ���Լռ�Թ��ݻ�����֮һ�ij���ʯ��ˮ��
�����������ϡ��³�����һ��ʱ�䡣
��д��ʵ���Ҽ���CO2�Ļ�ѧ����ʽ ��
�ڳ�����Թ���ѹǿ �Թ������ѹǿ(ѡ�>����=����<��)��
�����Թ�ǰ������������Ŀ���ǣ� �� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com