
| A�� | ��˿��������ȼ�գ�2Fe+3O2 $\frac{\underline{\;��ȼ\;}}{\;}$ 2Fe2O3 | |
| B�� | һ����̼��ԭ��������3CO+Fe2O3=2Fe+3CO2 | |
| C�� | ���ܱ�������ȼ�պ�����֤�����غ㶨�ɣ�2P+O2 $\frac{\underline{\;��ȼ\;}}{\;}$ P2O5 | |
| D�� | �ó����ʯ��ˮ���������̼���壺CO2+Ca��OH��2=CaCO3��+H2O |
���� ���ݻ�ѧ����ʽ�ж�����ķ����迼�ǣ�Ӧ�õ�ԭ���Ƿ���ȷ����ѧʽ��д�Ƿ���ȷ���Ƿ���ƽ����Ӧ�����Ƿ���ȷ�����͡��ı�ע�Ƿ���ȷ��
��� �⣺A����ѧ����ʽ�����Ͽ���ʵ�������ﻯѧʽд����Ӧ��Fe3O4����ȷ��ѧ����ʽΪ��3Fe+2O2$\frac{\underline{\;��ȼ\;}}{\;}$Fe3O4������
B��ȱ�ٷ�Ӧ����������ȷ�Ļ�ѧ����ʽΪ��3CO+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2������
C����ѧ����ʽû����ƽ����ȷ��ѧ����ʽΪ��4P+5O2 $\frac{\underline{\;��ȼ\;}}{\;}$ 2P2O5������
D���û�ѧ����ʽ��д��ȫ��ȷ��
��ѡD
���� ������Ҫ���黯ѧ����ʽ����д���������ɣ�����Ŀ�е���Ϣ����֪��Ӧ��������Ӧ��������д�˻�ѧ����ʽ�ķ�����


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 35% | B�� | 25% | C�� | 20% | D�� | 10% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ����� | �� | �� | �� | �� |
| ˮ��������g�� | 10 | 10 | 10 | 10 |
| ����NaCl��������g�� | 2 | 3 | 4 | 5 |
| ��Һ��������g�� | 12 | 13 | 13.6 | 13.6 |
| A�� | ����������Һ�DZ�����Һ | B�� | 20��ʱ10gˮ������ܽ�4gNaCl | ||
| C�� | �ۢ���Һ��Ũ����ͬ | D�� | ����Һ�Dz�������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
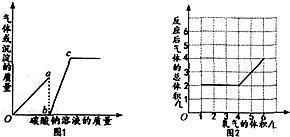
| A�� | ͼl��oa�α�ʾʵ���г������õı仯��� | |
| B�� | ͼl��c���ʾ���������Һ��̼������Һǡ����ȫ��Ӧ | |
| C�� | ��ͼ2��֪����ӦǰX�����Ϊ2L | |
| D�� | ��ͼ2��֪��x������C0��CH4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
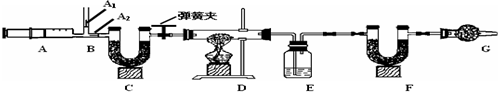
| ��Ӧǰ | ��Ӧ�� |
| E������Ϊ100.0g | E������Ϊ105.4g |
| F������Ϊ50.0g | F������Ϊ54.4g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | ����ϡ���������/�� | ʣ����������/�� |
| ��һ�� | 20 | 11 |
| �ڶ��� | 20 | 6 |
| ������ | 20 | 2.8 |
| ���Ĵ� | 20 | n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
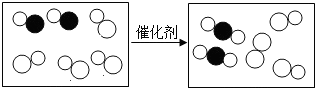
| A�� | �˷�Ӧ�е������� | |
| B�� | ԭ���ڻ�ѧ�仯���ǿɷֵ� | |
| C�� | �˷�Ӧ���������������� | |
| D�� | �μӷ�Ӧ�����ַ��ӵĸ�����Ϊ2��1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com