
| 第一次 | 第二次 | 第三次 | |
| 盐酸质量/g | 50 | 150 | 200 |
| 烧杯内剩余物质的质量/g | 100 | 197.8 | 245.6 |
| 现象 | 无气泡,溶液仍为红色 | 溶液中有气泡产生,溶液仍为红色 | 溶液中有气泡产生,溶液恰好由红色变为无色 |
| 106 |
| x |
| 73 |
| z |
| 117 |
| y |
| 44 |
| 4.4g |
| 7.3g |
| 7.3% |
| 36.5 |
| 100g×7.3% |
| 40 |
| q |
| 58.5 |
| p |
| 106 |
| 44 |
| x |
| 4.4g |
| 11.7g+11.7g |
| 245.6g+154.4g |
| 80 |
| 106 |
| w |
| 10.6g |
| 106 |
| 44 |
| x |
| 4.4g |


科目:初中化学 来源: 题型:
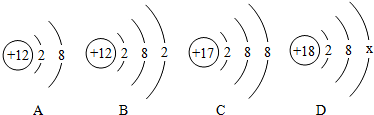
查看答案和解析>>
科目:初中化学 来源: 题型:
| 序号 | 物质 | 杂质 | 试剂 | 操作方法 |
| A | 盐酸 | 硫酸 | 适量的氯化钡溶液 | 过滤 |
| B | 氯化钙 | 碳酸钙 | 适量的稀盐酸 | 蒸发、结晶 |
| C | 二氧化碳 | 水蒸气 | 浓硫酸 | 洗气 |
| D | 硝酸钠溶液 | 碳酸钠 | 适量的氯化钙溶液 | 过滤 |
| A、A | B、B | C、C | D、D |
查看答案和解析>>
科目:初中化学 来源: 题型:
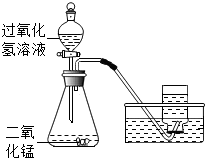
| 气体发生装置内物质的总质量 | |
| 反应前 | 36.4g |
| 反应后 | 34.8g |
查看答案和解析>>
科目:初中化学 来源: 题型:
查看答案和解析>>
科目:初中化学 来源: 题型:
| A、2Fe+3H2SO4═Fe2(SO4)3+3H2↑ | ||||
| B、Cu+AgNO3═Cu(NO3)2+Ag | ||||
C、CO+CuO
| ||||
| D、CaCO3+2HCl═CaCl2+H2O+CO2 |
查看答案和解析>>
科目:初中化学 来源: 题型:
| ||
查看答案和解析>>
科目:初中化学 来源: 题型:
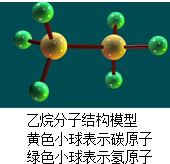
| A、乙烷由两种元素组成 | ||
| B、乙烷中碳、氢元素的质量之比为12:1 | ||
| C、乙烷分子中碳、氢原子个数之比为1:3 | ||
D、乙烷中氢元素的质量分数为
|
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com