
| 实验次数 项目 |
第一次 | 第二次 | 第三次 |
| 所取合金的质量/g | 20 | 20 | 40 |
| 所加稀硫酸的质量/g | 100 | 120 | 80 |
| 生成氢气的质量/g | 0.4 | 0.4 | 0.4 |
| 56 |
| 2 |
| 20x |
| 0.4 |
| 56 |
| 152 |
| 20×56% |
| y |
| 30.4g |
| 90.8g |


 名师金手指领衔课时系列答案
名师金手指领衔课时系列答案科目:初中化学 来源: 题型:
| 第一次 | 第二次 | 第三次 | |
| 所取合金的质量/g | 20 | 20 | 20 |
| 所加稀硫酸的质量/g | 100 | 120 | 80 |
| 生成氢气的质量/g | 0.4 | 0.4 | 0.4 |
查看答案和解析>>
科目:初中化学 来源: 题型:
| 实验次数 项目 |
第一次 | 第二次 | 第三次 | 第四次 |
| 所取合金的质量/g | 10 | 10 | 10 | 10 |
| 所加稀硫酸的质量/g | 40 | 60 | 80 | 100 |
| 生成氢气的质量/g | 0.1 | 0.15 | 0.2 | 0.2 |
查看答案和解析>>
科目:初中化学 来源: 题型:
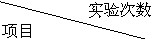 |
第一次 | 第二次 | 第三次 |
| 所取合金的质量/g | 20 | 20 | 40 |
| 所加稀硫酸的质量/g | 100 | 120 | 80 |
| 生成氢气的质量/g | 0.4 | 0.4 | 0.4 |
查看答案和解析>>
科目:初中化学 来源:2008-2009学年人教版九年级(下)第一次月考化学试卷(解析版) 题型:解答题
| 第一次 | 第二次 | 第三次 | |
| 所取合金的质量/g | 20 | 20 | 20 |
| 所加稀硫酸的质量/g | 100 | 120 | 80 |
| 生成氢气的质量/g | 0.4 | 0.4 | 0.4 |
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com