
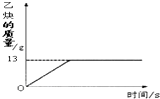
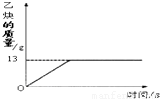
 ��1����Ȳ��C2H2����һ����Ҫ����ԭ�ϣ�ʵ���ҳ���̼���ƣ�CaC2����ˮ��Ӧ��ȡ��ij��ѧ��ȤС��ȡһ��������̼������90gˮ��Ӧ����������������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ����ش��������⣺
��1����Ȳ��C2H2����һ����Ҫ����ԭ�ϣ�ʵ���ҳ���̼���ƣ�CaC2����ˮ��Ӧ��ȡ��ij��ѧ��ȤС��ȡһ��������̼������90gˮ��Ӧ����������������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ����ش��������⣺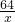 =
=
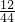 ��100%=12g��27gˮ��������Ԫ�ص�����=27g��
��100%=12g��27gˮ��������Ԫ�ص�����=27g��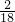 ��100%=6g���ɴ˿�֪���л�����̼����Ԫ�ص���������12g��6g=2��1���û�������̼����Ԫ�ص�������=12g+6g=18g��С���л��������32g������л����к���Ԫ�أ�������Ԫ�ص�����=32g-18g=14g��
��100%=6g���ɴ˿�֪���л�����̼����Ԫ�ص���������12g��6g=2��1���û�������̼����Ԫ�ص�������=12g+6g=18g��С���л��������32g������л����к���Ԫ�أ�������Ԫ�ص�����=32g-18g=14g��

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1����Ȳ��C2H2����һ����Ҫ����ԭ�ϣ�ʵ���ҳ���̼���ƣ�CaC2����ˮ��Ӧ��ȡ��ij��ѧ��ȤС��ȡһ��������̼������90gˮ��Ӧ����������������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ����ش��������⣺
��1����Ȳ��C2H2����һ����Ҫ����ԭ�ϣ�ʵ���ҳ���̼���ƣ�CaC2����ˮ��Ӧ��ȡ��ij��ѧ��ȤС��ȡһ��������̼������90gˮ��Ӧ����������������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ����ش��������⣺�鿴�𰸺ͽ���>>
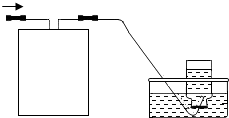
��Ŀ�����л�ѧ ��Դ��2012���Ĵ�ʡ��Ԫ�н����ؽ�����ѧ�п���ѧģ���Ծ��������������棩 ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���Ĵ�ʡ��Ԫ�н����ؽ�����ѧ�п���ѧģ���Ծ���һ���������棩 ���ͣ������
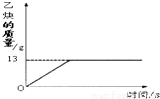
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com