

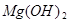 +2HCl=
+2HCl= +H2O��
+H2O��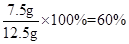 ��
��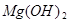 +2HCl=
+2HCl= +H2O
+H2O ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
 AS���ס��ҡ�������ѧ����ʵ���ҷֱ���и�ʵ�飬�����A���������������ͬ������������Ϊ12g�� �ֱ�Ӧʱ��ʵ�����ݼ�¼���£�
AS���ס��ҡ�������ѧ����ʵ���ҷֱ���и�ʵ�飬�����A���������������ͬ������������Ϊ12g�� �ֱ�Ӧʱ��ʵ�����ݼ�¼���£�| | A������/ g | S������/ g | AS������/ g |
| �� | 8 | 4 | 11 |
| �� | 7 | 5 | 11 |
| �� | a | b | 6.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ����ϡ��������� | 35g | 35g | 35g | 35g |
| ʣ���������� | 8.6g | 7.4g | 6.2g | 5.6g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1/32 g | B��1/35 g | C��1/64 g | D��1/70 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��48g���� | B��72g������ | C��144g�� | D��36g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| ���� | С�ڵ���15ǧ�� | 15��23ǧ�� | 23��40ǧ�� | ����40ǧ�� |
| ÿ������ | 30mg | 45mg | 60mg | 60mg |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com