
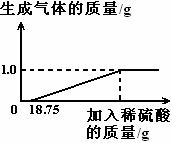
ij��ȤС�����������������ĺ��������˲ⶨ������������г�Fe��Fe2O3�⣬������̼���衢�̵�Ԫ�أ���Fe��Fe2O3��������ʶ�����ϡ���ᷴӦ������ȤС���ͬѧ��ȡ33g������Ƭ�������������У���������39.2%��ϡ���ᣬֱ����Ӧ��ȫ���������������������ⷢ����Ӧ����ʵ��������ͼ��ʾ��
��1���ӻ�ѧԪ�������彡���ĽǶȿ��ǣ�����Ӧѡ�������������� ��Ϊʲô ��
��2��������Ƭ�������ʵ���������Ϊ �������������0.1%��
��3��������Һ���������ʵĻ�ѧʽΪ ��������Һ����������������
��Ҫ��д��������̣�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?��Ӫ��ij��ȤС�����������������ĺ��������˲ⶨ������������г�Fe��Fe2O3�⣬������̼���衢�̵�Ԫ�أ���Fe��Fe2O3��������ʶ�����ϡ���ᷴӦ������ȤС���ͬѧ��ȡ33g������Ƭ�������������У���������39.2%��ϡ���ᣬֱ����Ӧ��ȫ���������������������ⷢ����Ӧ����ʵ��������ͼ��ʾ��
��2012?��Ӫ��ij��ȤС�����������������ĺ��������˲ⶨ������������г�Fe��Fe2O3�⣬������̼���衢�̵�Ԫ�أ���Fe��Fe2O3��������ʶ�����ϡ���ᷴӦ������ȤС���ͬѧ��ȡ33g������Ƭ�������������У���������39.2%��ϡ���ᣬֱ����Ӧ��ȫ���������������������ⷢ����Ӧ����ʵ��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ȤС�����������������ĺ��������˲ⶨ������������г�Fe��Fe2O3�⣬������̼���衢�̵�Ԫ�أ���Fe��Fe2O3��������ʶ�����ϡ���ᷴӦ������ȤС���ͬѧ��ȡ33g������Ƭ�������������У���������39.2%��ϡ���ᣬֱ����Ӧ��ȫ���������������������ⷢ����Ӧ����ʵ��������ͼ��ʾ��
ij��ȤС�����������������ĺ��������˲ⶨ������������г�Fe��Fe2O3�⣬������̼���衢�̵�Ԫ�أ���Fe��Fe2O3��������ʶ�����ϡ���ᷴӦ������ȤС���ͬѧ��ȡ33g������Ƭ�������������У���������39.2%��ϡ���ᣬֱ����Ӧ��ȫ���������������������ⷢ����Ӧ����ʵ��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ȤС�����������������ĺ��������˲ⶨ������������г�Fe��Fe2O3�⣬������̼���衢�̵�Ԫ�أ���Fe��Fe2O3��������ʶ�����ϡ���ᷴӦ������ȤС���ͬѧ��ȡ40 g������Ƭ�������������У���������39.2%��ϡ���ᣬֱ��ǡ�÷�Ӧ��ȫֹͣ����ϡ���ᣨ�������������������ⷢ����Ӧ����ʵ����������ͼ��ʾ��
��1��������Ƭ�������ʵ���������Ϊ ��
��2��������Һ���������ʵĻ�ѧʽΪ ���������
Һ��������������Ϊ g����д�������������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ��ҵ��ѧҵ���Ե�һ��Ԥ�⻯ѧ�Ծ��������棩 ���ͣ�������
ij��ȤС�����������������ĺ��������˲ⶨ������������г�Fe��Fe2O3�⣬������̼���衢�̵�Ԫ�أ���Fe��Fe2O3��������ʶ�����ϡ���ᷴӦ������ȤС���ͬѧ��ȡ33 g������Ƭ�������������У���������39.2%��ϡ���ᣬֱ����Ӧ��ȫ���������������������ⷢ����Ӧ����ʵ��������ͼ��ʾ��

��1���ӻ�ѧԪ�������彡���ĽǶȿ��ǣ�����Ӧ��ѡ��
��ѡ���������������������Ϊ ��
��2��������Ƭ�������ʵ���������Ϊ ���������ȷ��0.1%��
��3��������Һ���������ʵĻ�ѧʽΪ ��������Һ����������������
��Ҫ��д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ�п����� ���ͣ�������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com