
С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ������Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶ�����������ȡ16.25g������Ʒ��ȫ������143.6mLˮ�У������õ��Ļ����Һ����μ���������������Ϊ10.6%��̼������Һ���õ�����ͼ��ʾ�����߹�ϵ��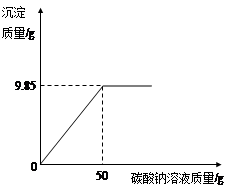
����Ա��С�����ʾ��
�ٷ�Ӧ�Ļ�ѧ����ʽ��BaCl2��Na2CO3��BaCO3����2NaCl
��ˮ���ܶȣ�1g/cm3
���Ʒ���Ȼ��Ƶ�����������
36%
����������������������ݴ����Ļ�ѧ����ʽ�����⣬����ͼ����֪���������ĵ�̼������Һ����Ϊ50gʱ����Ӧ��������ʱ���ɵ�̼�ᱵ��������Ϊ9.85g���ʿɸ��ݷ���ʽBaCl2��Na2CO3��BaCO3����2NaCl��̼�ᱵ��BaCl2��������ϵ�������BaCl2�������������������Ʒ�е��Ȼ��������������������Ʒ���Ȼ��Ƶ���������
�⣺����Ʒ��BaCl2������Ϊx
Na2CO3������BaCl2��BaCO3����2NaCl
106����������208
50g��10.6������x ��x��10.4g
��x��10.4g
��Ʒ��NaCl����������Ϊ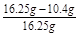 ��100%��36%
��100%��36%
�𣺲�Ʒ��NaCl����������Ϊ36%��
���㣺���ݻ�ѧ����ʽ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��1������200g��������10%���Ȼ�����Һ����Ҫ�����Ȼ��Ƶ�����Ϊ������ʽ���㣩
= g
(2) ij�������÷���м�ͷ�������Ӧ����ȡ�����������������з�����19��6�֣�H2SO4��������Ϊ10%�����������ķ���м��Ӧ�������������ٶ�������������Fe+H2SO4= FeSO4+H2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��3�֣��ѣ�Ti���ǹ㷺Ӧ���ں��ա������������һ����Ҫ��������һ�������£��������Ȼ��ѣ�TiCl4���ͽ���þ��Ӧ���Ƶã�TiCl4+2Mg==Ti+2MgCl2 ������380kg���Ȼ��ѣ���������������ѵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
Ϊ�˲ⶨδ֪Ũ��ϡ�������������������ȡ����������Һ15g����μ���ô���ϡ���ᣬͬʱ�ⶨ����¼��Ӧ�����л����Һ��pH�仯�������ͼ��ʾ�������˺�õ���Һ32��67g��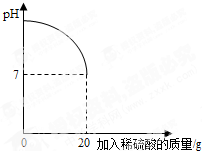
��1����Ӧ���������ᱵ�������Ƕ��٣�
��2����ϡ�������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ijѧ����������ͼ��ʾ��ʵ�顣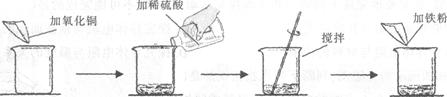
| | ��һ�� | �ڶ��� |
| ��������ͭ������ | m | m |
| ����ϡ��������� | 50g | 100g |
| �������۵����� | 5��6g | 5��6g |
| ʵ������ | ��ɫ������Ϻ�ɫ���� | �Ϻ�ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�Ҵ���һ��������ɫ��Դ�����������׳ƾƾ����仯ѧʽΪC2H5OH�����ڿ�������ȫȼ�յĻ�ѧ����ʽΪ��C2H5OH+3O2 2CO2+3H2O������100g��������Ϊ92%���Ҵ���Һ�ڿ�������ȫȼ�ղ���������̼������Ϊ���ٿˣ�
2CO2+3H2O������100g��������Ϊ92%���Ҵ���Һ�ڿ�������ȫȼ�ղ���������̼������Ϊ���ٿˣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�����£���49.8gijŨ�ȵ�����������Һ����ε���160g��������Ϊ10����CuSO4
��Һ������ǡ����ȫ��Ӧ���Լ��㣺
��1��������������Һ��NaOH������Ϊ g��У�ԣ���У��Դ��༭03
��2����Ӧ��������Һ�����ʵ����������Ƕ���?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ҵ���õ��ˮ�ķ�����ȡ��������ѧ����ʽΪ 2H2O 2H2��+ O2 ����
2H2��+ O2 ����
�Լ��㣺���7.2t��ˮ�����Ƶ������������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��������AlN�����㷺Ӧ���ڵ��ӹ�ҵ���մɹ�ҵ��������һ�������£���������ͨ�����·�Ӧ�Ƶã�Al2O3+N2+3C�T2AlN+3CO�������Ʊ�12.3t����������Ҫ�μӷ�Ӧ�ĵ�����N2�������Ƕ��٣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com