
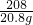 =
= x=23.3g
x=23.3g

 ЬсЗжАйЗжАйМьВтОэЕЅдЊЦкФЉВтЪдОэЯЕСаД№АИ
ЬсЗжАйЗжАйМьВтОэЕЅдЊЦкФЉВтЪдОэЯЕСаД№АИ аЁбЇЦкФЉБъзМЪдОэЯЕСаД№АИ
аЁбЇЦкФЉБъзМЪдОэЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК2008ФъЩНЖЋЪЁМУФЯЪаГѕжаБЯвЕЩ§бЇЭГвЛПМЪдЁЂЛЏбЇЪдОэ ЬтаЭЃК043
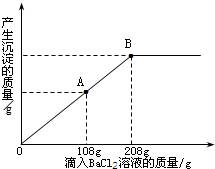
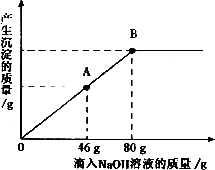
дквЛЩеБжаЪЂгавЛЖЈжЪСПЕФMgCO3ЙЬЬхЃЌЯђЦфжаЕЮМгШмжЪЕФжЪОЋЗжЪ§ЮЊ10ЃЅЕФH2SO4ШмвКЃЌжСЧЁКУЭъШЋЗДгІЃЎЕУЕН102 gВЛБЅКЭШмвКЃЎЯђЫљЕУШмвКжаж№ЕЮЕЮШЫШмжЪжЪСПЗжЪ§ЮЊl0ЃЅЕФNaOHШмвКЃЌВњЩњГСЕэЕФжЪСПгыЫљЕЮШыNaOHШмвКЕФжЪСПЙиЯЕЧњЯпШчЭМЫљЪОЃЎЧыИљОнЬтвтЛиД№ЯТСаЮЪЬтЃК
(1)дкЕЮШыЯЁСђЫсЪБЃЌЙлВьЕНЕФУїЯдЪЕбщЯжЯѓЪЧ________ЃЎ
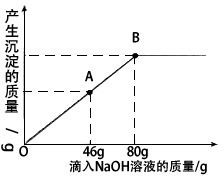
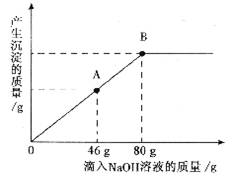
(2)ЕБЕЮШыNaOHШмвКжСЭМжаAЕуЪБЃЌЩеБжаШмвКРяКЌгаЕФШмжЪЪЧ(аДЛЏбЇЪН)________ЃЎ
(3)ЕБЕЮШы10ЃЅЕФNaOHШмвК80 gЪБ(МДBЕу)ЃЌЪдЭЈЙ§МЦЫуЃЌЧѓДЫЪБЫљЕУаЁБЅКЭШмвКЕФжЪСПЃЎ(МЦЫуНсЙћОЋШЗжС0.1 g)
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКГѕжаЛЏбЇ РДдДЃКЩНЖЋЪЁжаПМецЬт ЬтаЭЃКМЦЫуЬт
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК ЬтаЭЃК
дквЛЩеБжаЪЂгавЛЖЈжЪСПЕФMgCO3ЙЬЬхЃЌЯђЦфжаЕЮМгШмжЪЕФжЪОЋЗжЪ§ЮЊ10ЃЅЕФH2SO4ШмвКЃЌжСЧЁКУЭъШЋЗДгІЁЃЕУЕН102gВЛБЅКЭШмвКЁЃЯђЫљЕУШмвКжаж№ЕЮЕЮШЫШмжЪжЪСПЗжЪ§ЮЊl0ЃЅЕФNaOHШмвКЃЌВњЩњГСЕэЕФжЪСПгыЫљЕЮШыNaOHШмвКЕФжЪСПЙиЯЕЧњЯпШчЭМЫљЪОЁЃЧыИљОнЬтвтЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉдкЕЮШыЯЁСђЫсЪБЃЌЙлВьЕНЕФУїЯдЪЕбщЯжЯѓЪЧ______________________________ЁЃ
ЃЈ2ЃЉЕБЕЮШыNaOHШмвКжСЭМжаAЕуЪБЃЌЩеБжаШмвКРяКЌгаЕФШмжЪЪЧЃЈаДЛЏбЇЪНЃЉ ____________________________________________________________________________ЁЃ
ЃЈ3ЃЉЕБЕЮШы10ЃЅЕФNaOHШмвК80gЪБЃЈМДBЕуЃЉЃЌЪдЭЈЙ§МЦЫуЃЌЧѓДЫЪБЫљЕУаЁБЅКЭШмвКЕФжЪСПЁЃЃЈМЦЫуНсЙћОЋШЗжС0.1gЃЉ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com