
Сǿͬѧǰ�����ص�ʯ��ʯ�������е��飬��ȡ�������ɿ��ʯ��Ʒ������Ʒ��̼��Ƶ������������м�⣬�������°취��ȡ��8g����ʯ��ʯ��Ʒ����40gϡ�����4�μ��룬���������������ݼ��±�����֪ʯ��ʯ��Ʒ�к������ʲ�����ˮ���������ᷴӦ��������㣺����ѧ����ʽ��CaCO3 + 2HCl = CaCl2 + H2O + CO2����
��1��8gʯ��ʯ��Ʒ�к������ʶ��ٿˣ�
��2����Ʒ��̼��Ƶ����������Ƕ��٣�
��3���±���m����ֵӦ��Ϊ���٣�
| ��� | ����ϡ����������g�� | ʣ�����������g�� |
| ��1�� | 10 | 5.5 |
| ��2�� | 10 | m |
| ��3�� | 10 | 1.2 |
| ��4�� | 10 | 1.2 |
��4��Ҫ�õ�280kgCaO����Ҫ��������Ϊ80%��ʯ��ʯ����ǧ�ˣ�����ѧ����ʽ��CaCO3����==CaO+CO2����
������������ͨ�����ϸı��������ϡ������������۲�ʣ�������������ж�ϡ�����ʱ���㣬ʯ��ʯ��CaCO3��ʱ��ȫ��Ӧ���ɱ������ݿ�֪���ڵ����μ���10g�������ʣ�����������ټ��٣�˵��ʣ���1.2g���岻��ϡ���ᷴӦ��ӦΪ���ʡ�Ȼ����8gʯ��ʯ��Ʒ����������������CaCO3�������ٳ�����Ʒ���������������Ʒ��̼��Ƶ���������������С��Ҳ�ɴ�����ó����⣬����һ�μ�10g��ʱ�������������Ӧ�͵ڶ���һ�������Եڶ���ʣ��Ĺ�����������3g�����һ�ʿ����ú���������Ľ��ⷽ��������
���𰸡���1��8gʯ��ʯ��Ʒ�к�������Ϊ1.2g��
��2����Ʒ��̼��Ƶ�����������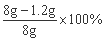 ��85��
��85��
��3��m��5.5g����8g��5.5g����3g
��4������Ҫ80%��ʯ��ʯ������Ϊx
CaCO3����==CaO+CO2��
100 56
x��80�� 280kg
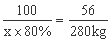 x��625kg
x��625kg


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | ����ϡ����������g�� | ʣ�����������g�� |
| ��1�� | 10 | 5.5 |
| ��2�� | 10 | m |
| ��3�� | 10 | 1.2 |
| ��4�� | 10 | 1.2 |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ����ϡ�����ʣ��ˣ� | 10 | 10 | 10 | 10 |
| ʣ������������ˣ� | 5.5 | M | 1.2 | 1.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | ����ϡ����������g�� | ʣ�����������g�� |
| ��1�� | 10 | 5.5 |
| ��2�� | 10 | m |
| ��3�� | 10 | 1.2 |
| ��4�� | 10 | 1.2 |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
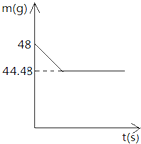 Сǿͬѧǰ�����ص�ʯ��ʯ�������е��飬��ȡ�������ɿ��ʯ��Ʒ������Ʒ��̼��Ƶ������������м�⣬���õİ취���£�ȡ��8g����ʯ��ʯ��Ʒ����40gϡ�����ַ�Ӧ����֪ʯ��ʯ��Ʒ�к������ʲ�����ˮ���������ᷴӦ��������ձ��еķ�Ӧʣ�����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ���Լ��㣺
Сǿͬѧǰ�����ص�ʯ��ʯ�������е��飬��ȡ�������ɿ��ʯ��Ʒ������Ʒ��̼��Ƶ������������м�⣬���õİ취���£�ȡ��8g����ʯ��ʯ��Ʒ����40gϡ�����ַ�Ӧ����֪ʯ��ʯ��Ʒ�к������ʲ�����ˮ���������ᷴӦ��������ձ��еķ�Ӧʣ�����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ���Լ��㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007�걱����˳�����п���ѧ��ģ�Ծ��������棩 ���ͣ������
| ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ����ϡ�����ʣ��ˣ� | 10 | 10 | 10 | 10 |
| ʣ������������ˣ� | 5.5 | M | 1.2 | 1.2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com