����ԭ������һ����Ҫ�Ļ���ԭ�ϣ�ij��ȤС�������������о���
�������Ʊ��������̷��Ʊ�����ԭ���۵Ĺ����������£�

FexOy+yH
2xFe+yH
2O Fe
3C+2H
23Fe+CH
4�ֻ�ԭ�����л��������������������Fe
3C���ʣ����������ڸ����½�һ����ԭ���䷴Ӧ����ʽΪ��
��
��1��д����������������CO��Ӧ�Ļ�ѧ����ʽ
��
��2�������мӽ�̿�����ó��˿�����ȼ���ṩ��Ӧ��������⣬����
��
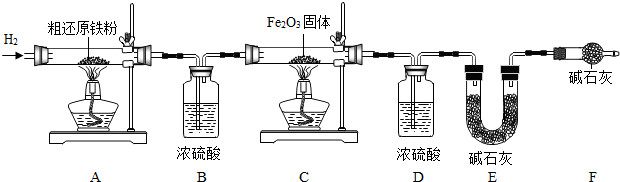
�������ⶨ����ͬѧΪ�õ�����ԭ���۲��ⶨ�ֻ�ԭ����������̼Ԫ�ص�����������������װ�ý���ʵ�飮
��֪��3CH
4+4Fe
2O
3 3CO
2+6H
2O+8Fe��Ũ��������ˮ�ԣ���ʯ�Ҳ���������ˮ����������CO
2��������ÿ����Ӧ����ȫ�Ҳ�����װ����ԭ�п����Բⶨ�����Ӱ�죩��
��3����Ҫʵ�鲽�����£�
�ٰ�˳����װ���������װ�õ������ԣ�������Ʒ�ͱ�Ҫװ�õ�������
�ڻ���ͨ�봿�������H
2��
�۵�ȼC���ƾ��ƣ�
�ܵ�ȼA ���ƾ��ƣ�
�ݷֱ�Ϩ��A��C���ƾ��ƣ�
��
��
���ٴγ�����Ҫװ�õ�������
��4������ڵ�Ŀ����
����֤�ò���Ŀ�Ĵﵽ��ʵ�鷽����
��
��5����Ӧ��Cװ���е�������
��
��6��װ��F��������
��
��7����ȱ��װ��D����������Ԫ�ص�����������
����ƫ����ƫС�����䡱����
��8��ʵ���ͬѧ����װ��
����A��B��C��D��E��F�������طֱ������Ʒ������̼Ԫ�ص�����������
��9����ͬѧ��10.0�˴ֻ�ԭ������Ʒ������ϡ�����ַ�Ӧ�����ⶨ������0.3�����壮����ôֻ�ԭ������Ʒ�������ʵ�������������Ҫ��д��������̣�