
 18”¢Ėį”¢¼ī”¢ŃĪŹĒÓŠ¹ć·ŗÓĆĶ¾µÄÖŲŅŖ»ÆŗĻĪļ£®
18”¢Ėį”¢¼ī”¢ŃĪŹĒÓŠ¹ć·ŗÓĆĶ¾µÄÖŲŅŖ»ÆŗĻĪļ£®

 ŹÖĄŹÖČ«ÓÅĮ·æ¼¾ķĻµĮŠ“š°ø
ŹÖĄŹÖČ«ÓÅĮ·æ¼¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
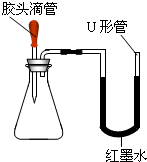 Ėį”¢¼ī”¢ŃĪŹĒÓŠ¹ć·ŗÓĆĶ¾µÄÖŲŅŖ»ÆŗĻĪļ£®Ä³»Æѧ»ī¶ÆŠ”×éµÄĶ¬Ń§Ī§ČĘÕā¼øĄą»ÆŗĻĪļ½ųŠŠĮĖŅ»ĻµĮŠµÄĢ½¾æ»ī¶Æ£®
Ėį”¢¼ī”¢ŃĪŹĒÓŠ¹ć·ŗÓĆĶ¾µÄÖŲŅŖ»ÆŗĻĪļ£®Ä³»Æѧ»ī¶ÆŠ”×éµÄĶ¬Ń§Ī§ČĘÕā¼øĄą»ÆŗĻĪļ½ųŠŠĮĖŅ»ĻµĮŠµÄĢ½¾æ»ī¶Æ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
²éæ““š°øŗĶ½āĪö>>
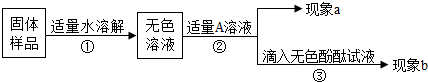
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ÅØĮņĖį£Ø·ÖĪö“棩 »ÆѧŹ½£ŗH2SO4 Ļą¶Ō·Ö×ÓÖŹĮæ£ŗ98 ĆÜ¶Č£ŗ1.84g/cm3 ÖŹĮæ·ÖŹż£ŗ98% |
| ŃōĄė×Ó\ŅõĄė×Ó | OH- | NO3- | Cl- | SO42- | CO32- |
| H+ | ČÜ”¢»Ó | ČÜ”¢»Ó | ČÜ | ČÜ”¢»Ó | |
| Na+ | ČÜ | ČÜ | ČÜ | ČÜ | ČÜ |
| Ba2+ | ČÜ | ČÜ | ČÜ | ²»ČÜ | ²»ČÜ |
| ŹµŃéÄæµÄ | ŹµŃé²Ł×÷ | ĻÖĻó | ½įĀŪ»ņ»Æѧ·½³ĢŹ½ |
³żČ„Ģ¼ĖįÄĘ |
ȔɣĮæøĆ¹ĢĢåѳʷČÜÓŚĖ®Åä³ÉČÜŅŗ£¬µĪ¼ÓŹŹĮæµÄ ³ä·Ö·“Ó¦ŗó¹żĀĖ |
ÓŠ°×É«³ĮµķÉś³É |
ÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ |
| ¼ģŃéŹĒ·ńŗ¬ÓŠĒāŃõ»ÆÄĘ | ŌŚĀĖŅŗÖŠµĪ¼Ó·ÓĢŖČÜŅŗ | øĆѳʷ֊ŗ¬ÓŠĒāŃõ»ÆÄĘ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2011ÄźŗÓÄĻŹ”æŖ·āŹŠÖŠæ¼»ÆѧŅ»Ä£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com