



 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NaCl��Na2CO3 |
| B��K2SO4��KCl |
| C��NH4NO3��NaOH |
| D��CuSO4��Fe2��SO4��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
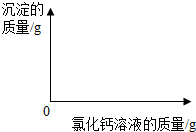 С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮
С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮| �ձ��� | �ձ��� | �ձ��� | |
| ����10.0%�Ȼ�����Һ��������g�� | 55.5 | 120.0 | 150.0 |
| ��ɫ�����������g�� | 5.0 | 10.0 | 10.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
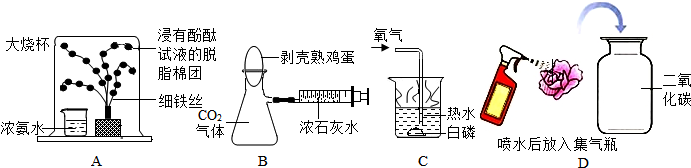
 С��ͬѧ��ѧϰ������ɷֲⶨʵ���Ľ��˿α��ϵ�װ��ͼ��ͼ���������ϵó����ͺ���һ���п�ȼ�ԣ���ȼ�պ��������Ҳ��ͬ����������40�漴��ȼ�գ��ش��������⣺
С��ͬѧ��ѧϰ������ɷֲⶨʵ���Ľ��˿α��ϵ�װ��ͼ��ͼ���������ϵó����ͺ���һ���п�ȼ�ԣ���ȼ�պ��������Ҳ��ͬ����������40�漴��ȼ�գ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com