
| ������ ������ | OH- | NO3- | Cl- | SO42- | CO32- |
| H+ | �ܡ��� | �ܡ��� | �� | �ܡ��� | |
| Na+ | �� | �� | �� | �� | �� |
| Ba2+ | �� | �� | �� | ���� | ���� |
| ʵ��Ŀ�� | ʵ����� | ���� | ���ۻ�ѧ����ʽ |
| ��ȥ̼���� | ȡ�����ù�����Ʒ�� ��ˮ�����Һ���μ������� | �а�ɫ�������� | �йط�Ӧ�Ļ�ѧ����ʽΪ |
| �����Ƿ��� �������� | ����Һ�еμӷ�̪��Һ | ����Ʒ�к����������� |
| 106 |
| x |
| 44 |
| 2.2g |
| 4.7g |
| 10g |
| ʵ��Ŀ�� | ʵ����� | ���� | ���ۻ�ѧ����ʽ |
| �Ȼ��� | Na2CO3+BaCl2=BaCO3��+2NaCl | ||
| ��ɫ���ɫ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
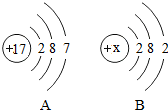
| A��B��ԭ�ӽṹʾ��ͼ��xΪ12 |
| B��A��B�ֱ����ڷǽ���Ԫ�غͽ���Ԫ�� |
| C��A��ԭ�Ӻ�B��ԭ�ӷֱ��γɼ����ӵĹ�����ͬ |
| D��A��B��ɵĻ������������ӹ��ɵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ���� | ��Ӧ�Ļ�ѧ����ʽ | |
| �����ϡ���� | ���� | ϡ���� | |
| þ | ��Ӧ | ||
| п | ��Ӧ ���� | | |
| �� | ��Ӧ | ||
| ͭ | \ | \ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ۢ� | B���ڢۢ� |
| C���ڢۢ� | D���٢ܢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com