

���� �������������������������������Ļ�ѧʽ���Լ���7.6��������������Ԫ�ص�������
������Ϣ��ʾ���Լ���FeSO4•7H2O����Է���������
��������Ҫ�ɷ����ܺ�ϡ���ᷴӦ�����������������������������������������Լ����������������ɵ�������������������һ�����Լ�����������������������
��� �⣺��1��7.6��������������Ԫ�ص�����Ϊ��7.6g��$\frac{56}{152}$��100%=2.8g��
��7.6��������������Ԫ�ص�����Ϊ2.8g��
��2��FeSO4•7H2O����Է�������Ϊ����56+32+16��4��+7����1��2+16��=278��
��FeSO4•7H2O����Է�������Ϊ278��
��3��������������Ϊx��������������������Ϊy��
Fe+H2SO4�TFeSO4+H2����
56 152 2
x y 0.2g
$\frac{56}{x}$=$\frac{152}{y}$=$\frac{2}{0.2g}$��
x=5.6g��y=15.2g��
��������15.2g����������
����������������������$\frac{5.6g}{6g}$��100%=93.3%��
��������������������Ϊ93.3%��
���� ������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�


 53������ϵ�д�
53������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A | ��ȫ��ʶ | B | ���ʵ�ȼ������ |
| �ٳ������Ż�ʱ���������Ϲ��� ����Ȼ��й©ʱ�������رշ��ſ���ͨ�� �ۼ��õ����Ż�ʱ��������ˮ���� | �ٺ�����������ȼ�ղ���Ũ������ ����˿�ڿ�����ȼ�գ��������䣬���ɺ�ɫ���� ������������ȼ�շ�������ɫ���棬�д̼�����ζ���������� | ||
| C | ���ʵ���������; | D | ��ѧ���� |
| ����Ϊ������֧��ȼ�գ����Կ�������ȼ���� ����Ϊ������ѧ���ʲ����ã����Կ���ʳƷ��װ���ڷ��� ����Ϊһ����̼���л�ԭ�ԣ����Թ�ҵ���������� | ��3����ԭ��-O3 ��2Fe3+����2����ʾ������������ ��$\stackrel{+2}{Mg}$����+2����ʾþԪ�صĻ��ϼ�Ϊ+2�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Թ����Һ�����ʱ�Թ���Һ�岻�����Թ��ݻ���1/3 | |
| B�� | ���ˮ�������븺���������������1/2 | |
| C�� | �����������뵪���������Լ��1/5 | |
| D�� | ���еĺ�̼����Ӧ�ó���2% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
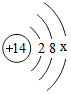 ����x=4��
����x=4���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  �ƾ���ʧ����ʪĨ���̸� | B�� |  ��ĥ���Ų����������ζ | ||
| C�� |  ����˫��ˮ��ȡ������ʣ���MnO2 | D�� | 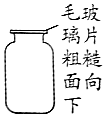 ����H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ȷ�� | B�� | ֻ�Т���ȷ�� | C�� | ֻ�Т���ȷ�� | D�� | ������ȷ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com