
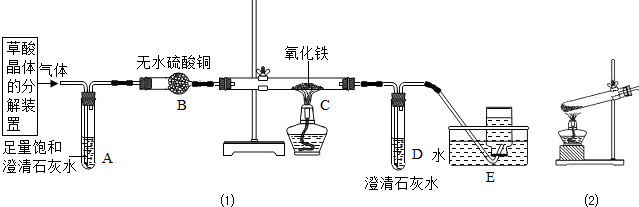
������ij��ѧ��ȤС��ʵʩ�Ĵ��� ��ء��Ȼ��ơ��Ȼ��صĻ����(�����Ȼ��ƺ��Ȼ��ص�������С������������3��)�з��������ص�ʵ�鲽�裺(�������ʵ��ܽ��������ͼ)
����������ƽ�Ƶ���Ʒ��������Ϊ87��5g��
�����Ƴ�80�����ҵı�����Һ��
���ȱ�����Һ��ȴ������(20��)����й��ˣ�����������
ˮϴ��2—3�Σ�
��ȡ���������еĹ��壬������װ��
��ش��������⣺
(1)��![]() ������ƽ��ȡ87��5g��Ʒʱ������Ӧ������ƽ��____�̣�
������ƽ��ȡ87��5g��Ʒʱ������Ӧ������ƽ��____�̣�
(2)ijͬѧ���֣����۽������ﻹ�����������������ʱ����ƽ
��������ƫת��ԭ����___________(�����)��
A����ƽδ������ˮƽ������ B����ƽû�е���
C�������µĵ�Ȧδȡ�� D������δ����
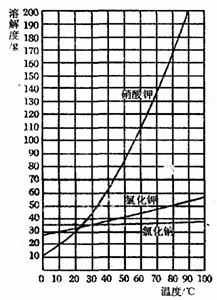
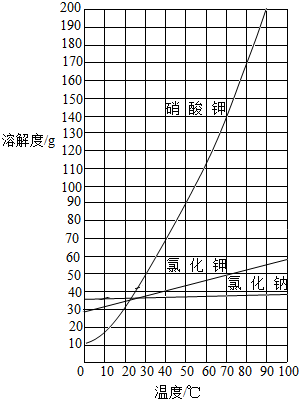
(3)����Щ��Ʒ�Ƴ�80�����ҵ��ȱ�����Һ��Լ��____ˮ(�����)��
A��12��5mL B��50mL C��100mL D��112��5mL
(4)��ʵ���У��������������ڽ���������⣬������_________________________��
(5)������У���������ˮϴ�ӹ����ԭ����____________________________________
_______________________________________________________________��
(6)���˲�ϴ�Ӻ��Ȼ��ش�����__________�У�
(7)���ʵ�����ȱ�����Һδ��ȫ��ȴ�����¾ͽ��й��˽���Ӱ������ع���IJ�����������_________________________________________________________________
![]() __________________________________________________________________��
__________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������ij��ѧ��ȤС��ʵʩ�Ĵ�����ء��Ȼ��ơ��Ȼ��صĻ��������Ȼ��ƺ��Ȼ��ص�������С����������3%���з��������ص�ʵ�鲽�裺���������ʵ��ܽ������ͼ��
������ij��ѧ��ȤС��ʵʩ�Ĵ�����ء��Ȼ��ơ��Ȼ��صĻ��������Ȼ��ƺ��Ȼ��ص�������С����������3%���з��������ص�ʵ�鲽�裺���������ʵ��ܽ������ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
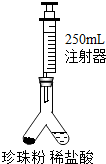 ��2013?������һģ��������������õ����յȹ�Ч�����������ϲ����������ʾ�������к�̼���80-93%��������4-14%��ˮ��2-4%��ʮ���ְ����ᡢ28����Ԫ�أ�Ȼ��һЩ���������û��յı��Ǽ��Ϲ�ҵ�������������Ƶ�ҩˮ������ϴ����ɹ�Ժ�ֱ�Ӽӹ����ۣ�����Ҫ�ɷ��ǣ�̼��ƣ������������������ƣ�������ij��ѧ��ȤС����Ʊ��������飬���ⶨ������̼��ƺ�����ʵ��̽��������
��2013?������һģ��������������õ����յȹ�Ч�����������ϲ����������ʾ�������к�̼���80-93%��������4-14%��ˮ��2-4%��ʮ���ְ����ᡢ28����Ԫ�أ�Ȼ��һЩ���������û��յı��Ǽ��Ϲ�ҵ�������������Ƶ�ҩˮ������ϴ����ɹ�Ժ�ֱ�Ӽӹ����ۣ�����Ҫ�ɷ��ǣ�̼��ƣ������������������ƣ�������ij��ѧ��ȤС����Ʊ��������飬���ⶨ������̼��ƺ�����ʵ��̽���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2013?��������ģ�����ʵ�鷽���ǿ�ѧ̽������Ҫ���ڣ�������ij��ѧ��ȤС����Ƶ��ĸ�ʵ�鷽�������з���һ�����������������ǣ�������
|
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com