�����Ǹ���ı��⣬Ϊ�����ṩ�˱������Ȼ��Դ��
��1��ʳ�ú�����ȡ����ҪӪ������
������
������
��
��2����ͼ1Ϊ��ˮ����װ�ã����õ���Դ��
̫����
̫����
����õ�����ˮ����
B
B
��
A������ B�������� C�������
��3�����ú�ˮ����ȡ�������Ʒ���ú�ˮ��ʳ�Σ���ͨ����ˮɹ�εõ����Σ������к���������CaCl
2��Na
2SO
4�����ʣ���ȥ�������ʣ��ȼӹ�����BaCl
2��Һ��ȥ���ټӹ���
Na2CO3
Na2CO3
��Һ��ȥCaCl
2������BaCl
2�����ɵij�����
CaCO3��BaCO3
CaCO3��BaCO3
��ȥ��������
ϡ����
ϡ����
����pH���������ᾧ���Ƶô��Σ�
��4�����Ȼ��ƺ�̼����刺��Ʊ�̼�����ƺ��Ȼ�泥��÷�Ӧ��ѧ����ʽ�ɱ�ʾΪ��NaCl+NH
4HCO
3=NaHCO
3+NH
4Cl.20��ʱ����������ѧ����ʽ�з�Ӧ��������ȣ���100��ˮ�м���11.7��NaCl��15.8��NH
4HCO
3�������ϴ���Һ��������������Ϊ
7.2
7.2
g��
���ϣ�20��ʱ�������ʵ��ܽ�����£���������ͬʱ�ܽ���ˮ�и��Ե��ܽ�Ȳ��䣬
| ���� |
NaCl |
NH4CO3 |
NH4Cl |
NaHCO3 |
| �ܽ�� |
36.0 |
21.6 |
37.2 |
9.6 |
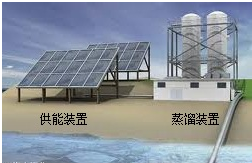
��5���Ӻ�ˮ����ȡ�峣�ô�������������������ˮ����廯���е����û����������ÿ�����ˮ���������壮����ʾ��ͼ��ͼ2��
��д�������û����嵥�ʵĻ�ѧ����ʽ��
Cl2+2NaBr=2NaCl+Br2
Cl2+2NaBr=2NaCl+Br2
���÷�Ӧ��pH=3�����������½��У�����
pH��ֽ
pH��ֽ
�ⶨ��ӦҺ�����ȣ�
�ڴ�������ʹ������������һ��������������������з����ķ�Ӧ��Br
2+SO
2+2H
2O?H
2SO
4+2HBr������������ͼ������ͨ��SO
2��Ŀ����
�����������
�����������
��





 ��2012?����������Ϊ�����ṩ�˱������Ȼ��Դ��
��2012?����������Ϊ�����ṩ�˱������Ȼ��Դ��