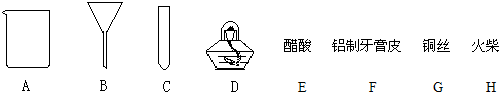
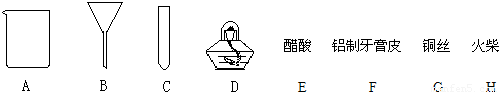
�⣺��1���Գ���������ʶ�ǣ���Ϊ���ձ����ƾ���
��2�����ý������ᷴӦ�Ϳ������������ʴ�Ϊ��EF
��3��a���������п�����������ᷢ����ը�����Ե�ȼ����ǰҪ�鴿���ʴ�Ϊ�����������ȼ���
b����Ϊ�������ܶȱȿ���С�����������ſ������ռ����ʴ�Ϊ�������ſ�����
��4��a��������֪��ͭ�ڿ����м������ɺ�ɫ������ͭ������ͭ�������м��ȿ�����ͭ��ˮ���ʴ�Ϊ��2Cu+O
2
2CuO��H
2+CuO

Cu+H
2O
b����ͭ˿���H
2ʱ��Ϊ�˳�ַ�Ӧ��Ӧ��ͭ˿���������룬�ʴ�Ϊ����������������
c����ͭ˿����������״��Ŀ���������������ĽӴ�������ʴ�Ϊ�������������ĽӴ����
��ʵ��H
2+CuO

Cu+H
2O֪�������ܶ�ȡ����ͭ������������ǻ�ԭ�����ʴ�Ϊ����ԭ��
��������1�����ݳ���������ʶ�ǽ��н��
��2������ʵ������ȡ������ԭ��������
��3�����������Ŀ�ȼ�Ժ��ܶȽ��н��
��4�����ݷ�Ӧ��������P�����غ㶨��д������ʽ������ʵ���ע��������н���������Ŀ��
������������Ҫ������������ȡ���鴿����ԭ�ԣ����������������ķ�Ӧ��ѧ���̵���д��

 �ɺ�ɫ��ع����ĺ�ɫ��
�ɺ�ɫ��ع����ĺ�ɫ�� 2CuO��H2+CuO
2CuO��H2+CuO Cu+H2O
Cu+H2O Cu+H2O֪�������ܶ�ȡ����ͭ������������ǻ�ԭ�����ʴ�Ϊ����ԭ��
Cu+H2O֪�������ܶ�ȡ����ͭ������������ǻ�ԭ�����ʴ�Ϊ����ԭ��


 �ɺ�ɫ��ع����ĺ�ɫ��
�ɺ�ɫ��ع����ĺ�ɫ��