
����ˮ������Զ�س������Թ�����������ˮˮԴ��֮һ��ijУ������ѧ��ȤС���ͬѧ��ˮ��ˮ�ʵ�״����������صĵ����о�������������У�
��1��ȡ��ˮ�������ú���ˡ���ʵ�������Ҫ��ȥ��Һ���������ʣ�����Ҫ���е�ʵ���� ��
��2����Ҫ�ⶨ����ˮ���ˮ�����ȣ������˵��ǣ�����ĸ) ��
A����ɫʯ����Һ B��pH��ֽ C����ɫ��̪��Һ
��3����Ҫȡˮ�����ȣ���ȡˮ������������Թ��ݻ��� ��
��4��ȡ�������˺��ˮ�����������м������ɺ����н϶�Ĺ���������һ���������ֲ������к��н϶�Ca2+��˵������ˮ���ˮ���� ��
��5���ڻ�У�ijͬѧ��С�ı�����ҧ��(���϶�Һ������)������������������ͿĨ���Լ���ʹ����ǣ�����ĸ�� ��
A��ʳ�� B������Һ C����ˮ
��6��Ϊ����ˮԴ�أ���ֹˮ��Ⱦ���������һ�����������飺 ��
��1������ ��2��B ��3�� 1/3 ��4��Ӳˮ ��5��B ��6����ֹ����������
���������������1��������Գ�ȥˮ�еĿ��������ʣ�ȡ��ˮ�������ú���ˣ���ʵ�������Ҫ��ȥ��Һ���������ʣ�����Ҫ���е�ʵ��������
��2��pH��ֽ���Բⶨ��Һ�����ȣ���A��Cֻ�ܲⶨ��Һ������ԣ���Ҫ�ⶨ�����ҵ�ˮ�����ȣ�������pH��ֽ��
��3�����Թ��е�Һ�����ʱ��Ϊ��ֹҺ���������Թ�ʢҺ�岻�ܳ����ܼ��� ��
��
��4�����н϶�����Ըơ�þ�������ˮ��Ӳˮ������ˮ���ˮ�к��н϶�Ca2+��˵������ˮ���ˮ����Ӳˮ��
��5�����ϵ����Զ�Һ�������ʹ��ԭ��ͿĨ���Ե����ʰ��������ʷ�Ӧ���Ϳ��Լ�����ʹ��ABC��ֻ��B����ˮ�Ǽ��Եģ���ѡB��
��6��Ϊ����ˮԴ�أ���ֹˮ��Ⱦ����ҵ��ˮ���������������ŷţ�ũҵ����ү����ũҩ����ֹ����������.
���㣺ˮ�ľ��������Թ����Һ����ȣ����˵�ԭ������������Ӧ�ã���Һ�����Ȳⶨ��ˮ��Դ����Ⱦ����Σ��кͷ�Ӧ����Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣� ���������������ʣ�����ء�С�մ�����ʯī����Ȼ������������������Ҫ���û�ѧʽ��գ�
��1���ɳ���ʳƷ��װ�����ڷ������� �� ��2�����ͷ۵���Ҫ�ɷ�֮һ�� ��
��3�������ɵ�ص缫���� �� ��4������ȼ�ϣ�����Ҫ�ɷ��� ��
��5�������Ԫ���ǵؿ��к�����ߵĽ���Ԫ�� ����6���������Ϸ��ϵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣���ѧ������������ء�������صĻ�ѧ֪ʶ�ش��������⣺
��1�����������������ཡ�������봶�ߣ���Ҫԭ���� ������������ˮ��
�ȷ�����Ӧ�����⣬д��һ��Ԥ����������ľ������� ��
��2���ٽ��п�������Ϊ�˸�С������Ӫ�����ƶ������ʳ�����£�����ը���ȡ������㡢ţ�̡�ʳ���и��������ʳ���� ��Ϊ��Ӫ�����⣬�㽨��С�������軹Ӧ���ӵ�ʳ���� ��дһ�֣���
��3����������ҹ���س���������������PM2.5��������������ġ����ס�֮һ��
��PM2.5��ָ������ֱ��С�ڻ����2.5�Ŀ����Ҳ�ƿ���ο����������Ϊ�����PM2.5���� ��
| A����У�������ѻ��������͵ط��� | B��Ϊ������У��ᳫ���������ʹ��˽�ҳ� |
| C��������չ�������� | D��������ȼ�ű��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013 �������������ͻȪ��������������̶�����Ǻӵ�������Դ���齨���µ�һȪ�羰�����������ص���Ȼɽˮ���ۺ�������ʷ�Ļ�������һ�壬Ϊ 5A ��������
��1����ͻȪˮ����__________����������������
��2��ij��ѧС���ͬѧ�Ի��Ǻӵ�ˮ����������ص��о�����Ҫ�� pH ��ֽ���Բⶨ��ˮ�������ǿ�����ⶨ�ľ��巽����
��3��Ϊ�˼��������ˮ����ˮ����Ӳˮ������ˮ���м���___________�����衣
��4��������Ȫˮ֮������Ȫˮ������˵���д������ ������ţ�
| A���峺��Ȫˮ����Һ | B�������Ǿ����̶���ߵľ�ˮ���� |
| C��������еķ�������Ȫˮ��Ӳ�� | D�����˿��Գ�ȥȪˮ�еĿ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣�ˮ����Ҫ����Ȼ��Դ��������������������ʻ�����
��1��ˮ�Ǻܺõ��ܼ�������������ˮ��ϲ����γ���Һ���� ������ĸ��ţ���
A������ B��ʳ�� C��ֲ���� D���ƾ�
��2��ˮ��Һ��Ӧ�÷dz��㷺��3%�Ĺ���������Һ����������������3%�Ĺ�����
����Һ500g����Ҫ30% �Ĺ���������Һ g
��3����Ȼˮ�ǻ�������ͼ��ʾ�ļ���װ�þ���ˮʱ������̿����Ҫ������ �����н϶��Ca2+��Mg2+��ˮ����ΪӲˮ������Ӳˮ����ˮ�ķ����� ��Ӳˮ���ʱ���ˮ����ˮ������Ҫ�ɷ�Ϊ̼��ƺ�������þ����ʳ�׳�ˮ��ʱ�������ķ�ӦΪMg��OH��2 + 2CH3COOH ===��CH3COO��2Mg + 2H2O �� ��
��4��Ӣ����ѧ����ǰ���Ƴ�һ�����С���ˮ�������ʣ���ͼ����ÿ������ˮ����������Сˮ�Ρ�������ɳ�ʹ�Ĥ��ɵġ������Ƿ�״���ʵ������ɫ�Ǿ���ǿ�����ն�����̼�����������м��ߵ���ҵ��ֵ�������йء���ˮ����������ȷ���� ������ĸ��ţ���
A������ˮ�����ڴ����� B������ˮ���Ļ�ѧʽΪH2O
C������ˮ�������ڽ�������ЧӦ D������ˮ����һ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�ˮ��һ����������������ģ�����Ӧ���˽��й�ˮ��һЩ֪ʶ��
��1������ˮ�������䰮ˮ��Դ����ÿ������Ӧ�������κ������������������ڱ���ˮ��Դ���� (�����)��
A������ʹ�û���ũҩ B����ҵ��ˮ���������ŷ�
C��ʹ�ú���ϴ�·� D��������ˮֱ���ŷ�
��2��ˮҲ��������ܼ������������ʼ���������ˮ�У����γ���ɫ��Һ���� ��������ĸ��ţ�
A��ֲ���� B������ C��CaCO3 D���������
��3���ܽ��˽϶�ĵĿ����Ըƺ�þ�Ļ������ˮ����Ӳˮ��Ӳˮ������������в���Ӱ�졣�����У�һ����� �ķ�����ʹӲˮת��Ϊ��ˮ��
��4���ҹ����Ƴ���Ư�۸���Ч������ˮ��������ClO2������ȡClO2��ӦΪ��
X + 2NaClO2 ="=" 2ClO2 + 2NaCl����X�Ļ�ѧʽΪ ��
��5��д��һ����ˮ�μӵĻ��Ϸ�Ӧ�Ļ�ѧ��Ӧ����ʽ�� ��
��6����ʵ������Ũ��������ϡ����ʱ����Ҫ�õ�ˮ������Ҫ�����У����㡢 �����ȡ���ȴ������װƿ�����ϱ�ǩ�� ��Ҫ����184g��������Ϊ10%��ϡ���ᣬ��Ҫ��������Ϊ98%��Ũ����(�ܶ�Ϊ1.84 g/cm3) mL(����������һλС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����벻��ˮ������ˮ������ͨ�������������� �����þ�ˮ����װ�л���̿������Ϊ������ �ԡ�ͼһ�ǵ��ˮ��ʵ��װ�ã���ԴA���� �����˷�Ӧ�Ļ�ѧ����ʽΪ ��ͼ����ˮ������ʾ��ͼ������ȷ��ʾ��Ӧ���̵�˳���� ������Ԫ��������̬���ڵ�ͼʾ�� ������дͼʾ��ţ���ʵ����Եó����½��ۣ� ��
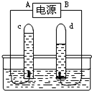 |  | ||||
| ͼһ | ͼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС���ͬѧ�������ͼ��ʾ�ļ��ĵ��ˮʵ��װ�ã����м�ͥСʵ�飬������������ݳ�������������±���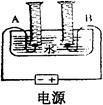
| ʱ�� | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| ���ӵ�Դ��������ͲA������ | 6 | 12 | 18 | 24 | 30 | 36 | 42 | 48 |
| ���ӵ�Դ��������ͲB������ | 2 | 4 | 6 | 9 | 12 | 15 | 18 | 21 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����벻��ˮ
��1�������У�����Ӳˮ����ˮ�� ��������������ˮ���м����Ҷ�����Ŀ���� ��
��2��ʵ���ҵ��ˮ����Դ���������������� ���ѧʽ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com