
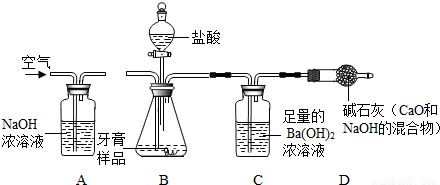
 =
=
 ×100%=25%��
×100%=25%��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ�鲽�� | ʵ������ | ��������� |
| ȡ������ҺA��ε���һ��������������Һ | ��ʼ���������������ɫ���� | ԭ���� HCl+NaOH=NaCl+H2O HCl+NaOH=NaCl+H2O ��AlCl3+3NaOH=Al��OH��3��+3NaCl AlCl3+3NaOH=Al��OH��3��+3NaCl �����û�ѧ����ʽ��ʾ�� |
| ������������������Һֱ������ | �����ܽ� �����ܽ� |
Ħ�����л����� �������� �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�걱���к��������꼶��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�̽����
ijͬѧ��̽������ ��˫�Ʒ��������ࡢ����塱��������۵���Ҫ�ɷ֡�

���������ϡ�
��1����������۾�����Ħ���������Լ������ϵȳɷֹ��ɡ�
��2�����õ�Ħ�����м�ϸ������̼���(CaCO3) ��ˮ�Ϲ���(SiO2��nH2O)��
��3������������г���̼������⣬�������ʾ�����ϡ���ᷴӦ�������塣
��ʵ��һ��̽��������������������Ƿ���̼��ƣ�
|
���� |
ʵ����� |
ʵ������ |
|
�� |
��ͼ��ʾ��ȡ��ֻ�Թֱܷ��������������Ʒ���ٷֱ��������R ��Һ��R ��Һ�� �� |
A�Թ��������Ա仯�� B��C�Թ�������ɫ�������ɡ� |
|
�� |
��B��C�Թ������ɵ���ɫ����ͨ�����ʯ��ˮ�� |
����� �� |
ʵ����ۣ� ��
��ʵ������Ƚ���Ʒ��̼��Ƶĺ���
|
���� |
ʵ����� |
ʵ������ |
|
�� |
��װ������ͼװ�ý���ʵ�顣�ֱ�ȡ ��������������ƿ�С����ڷ�Һ©���зֱ�����������R ��Һ��
|
|
|
�� |
��Һ©��ע��һ����R ��Һ��Ȼ��رջ����� |
�����ݲ����� |
|
�� |
���ڷ�Ӧ�������ٴ�Һ©����ע��һ����R ��Һ��Ȼ��رջ����� |
���������� |
|
�� |
������Ӧ��װ�ü�ҩƷ�������������ԱȽ� |
���롰˫�Ʒ����������װ�ü�ҩƷ�����������ڼ������۵�װ�ü�ҩƷ�������� |
ʵ����ۣ�_____________________________________________________
����۵������ǣ� ��
����˼��
��1�������Ķ�ԭ��װ�úͻ������裬Ҫ�ⶨ������̼��Ƶ�������������Ӧ�ⶨ�������У�_______________________________________________________________
��2��С����Ϊ��ʵ��ǰ����Ҫ�ⶨװ�õ������ԣ�����˵����ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ijͬѧ��̽������ ��˫�Ʒ��������ࡢ����塱��������۵���Ҫ�ɷ֡�
 ���������ϡ�
���������ϡ�
��1����������۾�����Ħ���������Լ������ϵȳɷֹ��ɡ�
��2�����õ�Ħ�����м�ϸ������̼���(CaCO3) ��ˮ�Ϲ���(SiO2��nH2O)��
��3������������г���̼������⣬�������ʾ�����ϡ���ᷴӦ�������塣
��ʵ��һ��̽��������������������Ƿ���̼��ƣ�
| ���� | ʵ����� | ʵ������ |
| �� |
��ͼ��ʾ��ȡ��ֻ�Թֱܷ��������������Ʒ���ٷֱ��������R ��Һ��R ��Һ�� �� | A�Թ��������Ա仯�� B��C�Թ�������ɫ�������ɡ� |
| �� | ��B��C�Թ������ɵ���ɫ����ͨ�����ʯ��ˮ�� | ����� �� |
ʵ����ۣ� ��
�� ʵ ��� ���Ƚ���Ʒ��̼��Ƶĺ���
��� ���Ƚ���Ʒ��̼��Ƶĺ���
| ���� | ʵ����� | ʵ������ |
| �� |
��������������ƿ�С����ڷ�Һ©���зֱ�����������R ��Һ�� | |
| �� | ��Һ©��ע��һ����R ��Һ��Ȼ��رջ����� | �����ݲ����� |
| �� | ���ڷ�Ӧ�������ٴ�Һ©����ע��һ����R ��Һ��Ȼ��رջ����� | ���������� |
| �� | ������Ӧ��װ�ü�ҩƷ�������������ԱȽ� | ���롰˫�Ʒ����������װ�ü�ҩƷ�����������ڼ������۵�װ�ü�ҩƷ�������� |
ʵ����ۣ�_____________________________________________________
����۵������ǣ� ��
���� ˼��
��1�������Ķ�ԭ��װ�úͻ������裬Ҫ�ⶨ������̼��Ƶ�������������Ӧ�ⶨ�������У�_______________________________________________________________
��2��С����Ϊ��ʵ��ǰ����Ҫ�ⶨװ�õ������ԣ�����˵����ԭ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com