
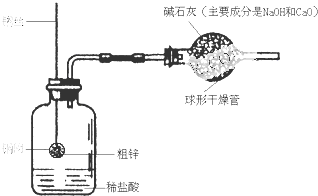
 ijͬѧ�����һ������ͼ��ʾ��װ�ã����ø�װ�òⶨ��п��Ʒ�ĺ�п����
ijͬѧ�����һ������ͼ��ʾ��װ�ã����ø�װ�òⶨ��п��Ʒ�ĺ�п����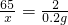
 ��100%=65%
��100%=65%

 Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
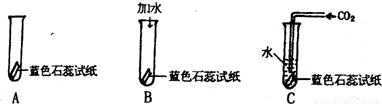
ijͬѧ�����һ����֤̼��������Զ�������̼û�����Ե�ʵ�飬ʵ�鰴����ͼʾ�ֱ���У� �ش��������⣺
����ɫʯ����ֽ��������Һ�� ɫ��
����Bʵ���У�������ˮ����ɫʯ����ֽ�� ɫ��Bʵ��������� ��
����Cʵ���У���ɫʯ����ֽ�� ɫ����һʵ����˵�� ��
������ʵ����δ�ﵽ��֤Ŀ�ģ���Ҫ�ﵽʵ��Ŀ�ģ���ĸĽ������� ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�����˽̰���꼶�ϲᡶ��6�� ̼��̼�������2013�굥Ԫ���ѵ����A��һ���������棩 ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2006-2007ѧ�긣��ʡ������ƽ��һ�о��꼶���ϣ��ڶ����¿���ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com