
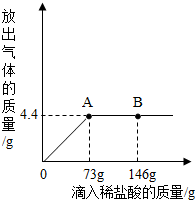
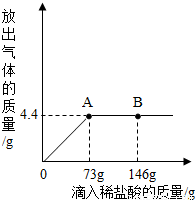
 һ�ձ���ʢ��9.3g NaHCO3��NaCl��ɵĹ��������57.1gˮ�ܽ⣬�Ƴ���Һ���������μ�������������Ϊ5%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ����NaHCO3+HCl�TNaCl+CO2��+H2O��
һ�ձ���ʢ��9.3g NaHCO3��NaCl��ɵĹ��������57.1gˮ�ܽ⣬�Ƴ���Һ���������μ�������������Ϊ5%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ����NaHCO3+HCl�TNaCl+CO2��+H2O�� ��
��
 ��100%=5%��
��100%=5%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
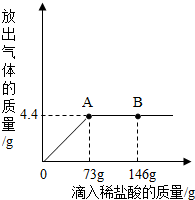 һ�ձ���ʢ��9.3g NaHCO3��NaCl��ɵĹ��������57.1gˮ�ܽ⣬�Ƴ���Һ���������μ�������������Ϊ5%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ����NaHCO3+HCl�TNaCl+CO2��+H2O��
һ�ձ���ʢ��9.3g NaHCO3��NaCl��ɵĹ��������57.1gˮ�ܽ⣬�Ƴ���Һ���������μ�������������Ϊ5%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ����NaHCO3+HCl�TNaCl+CO2��+H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
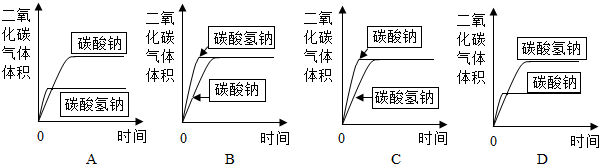
 ��2012?��¥��һģ���о���ѧϰС���̼���ƺ�̼�����Ƶ����ʽ���̽�����������ʵ�飮
��2012?��¥��һģ���о���ѧϰС���̼���ƺ�̼�����Ƶ����ʽ���̽�����������ʵ�飮| ̼����+���� | ̼������+���� | |
| ʵ������ | ||
| ��ѧ��Ӧ����ʽ |
�¶� �ܽ�� |
0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 100�� |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
| NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | - | - | - | - |
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | - |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ������������ѧ���꼶���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com