ŅŌĻĀŹĒĢ½¾æĖ®µÄ×é³ÉµÄŹµŃ飮ĻĀĶ¼ŹĒµē½āĖ®ŹµŃéµÄŹ¾ŅāĶ¼£ŗ
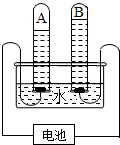
£Ø1£©Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½
£»
£Ø2£©A¶Ė½Óµē³Ų
¼«£ØĢī”°Õż”±»ņ”°øŗ”±£©£»
£Ø3£©µē½āĖ®µÄ¹ż³ĢÖŠ£¬·¢ÉśøıäµÄĪ¢Į£ŹĒ£ØŠ“Ćū³Ę£©
£»
£Ø4£©øĆŹµŃéæÉÖ¤Ć÷Ė®µÄŌŖĖŲ×é³É£¬Ė®ŹĒÓɣ؊“Ćū³Ę£©
×é³ÉµÄ£»
£Ø5£©Ė®ÖŠŌŖĖŲ“ęŌŚŠĪŹ½ŹĒ
£ØĢī”°ÓĪĄėĢ¬”±»ņ”°»ÆŗĻĢ¬”±£©£»
£Ø6£©¼ģŃéBŹŌ¹ÜÖŠĘųĢåµÄ²Ł×÷·½·ØŗĶĻÖĻóŹĒ
£»
£Ø7£©Čōµē½āĖ®ĻūŗÄĖ®3.6g£¬ŌņAŹŌ¹ÜÖŠÉś³ÉĘųĢåµÄ·Ö×ÓøöŹżŌ¼ĪŖ
£»
£Ø8£©ĪŖĮĖ½ųŅ»²½²ā¶ØĖ®ÖŠµÄŌŖĖŲ×é³ÉµÄÖŹĮæ±Č£¬Ä³æĘ¼¼Š”×éµÄĶ¬Ń§Éč¼ĘĮĖĻĀĮŠŹµŃé£Ø×°ÖĆČēĶ¼£©£¬
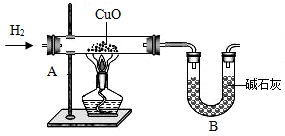
Ķعż³ĘĮæ·“Ó¦Ē°ŗó×°ÖĆA”¢BµÄÖŹĮ棬½į¹ū²āµĆm£ØH£©£ŗm£ØO£©£¾1£ŗ8£¬±ČĄķĀŪֵʫøߣ¬ĘäŌŅņæÉÄÜŹĒ
£®£ØĢī±ąŗÅ£©
A£®ĶØČėµÄĒāĘų¾¹ż¾»»ÆøÉŌļB£®×°ÖĆAÄŚ¹ÜæŚÓŠĖ®Äż½į
C£®Ńõ»ÆĶƻӊĶźČ«»¹ŌD£®×°ÖĆBĶ¬Ź±ĪüŹÕĮĖæÕĘųÖŠµÄĖ®ÕōĘųŗĶCO
2£®





 Ņ»ÅµŹéŅµŹī¼Ł×÷ŅµæģĄÖ¼ŁĘŚŌĘÄĻĆĄŹõ³ö°ęÉēĻµĮŠ“š°ø
Ņ»ÅµŹéŅµŹī¼Ł×÷ŅµæģĄÖ¼ŁĘŚŌĘÄĻĆĄŹõ³ö°ęÉēĻµĮŠ“š°ø
 £Ø2012?½õ½Ēų¶žÄ££©ŅŌĻĀŹĒĢ½¾æĖ®µÄ×é³ÉµÄŹµŃ飮ČēĶ¼ŹĒµē½āĖ®ŹµŃéµÄŹ¾ŅāĶ¼£ŗ
£Ø2012?½õ½Ēų¶žÄ££©ŅŌĻĀŹĒĢ½¾æĖ®µÄ×é³ÉµÄŹµŃ飮ČēĶ¼ŹĒµē½āĖ®ŹµŃéµÄŹ¾ŅāĶ¼£ŗ



