
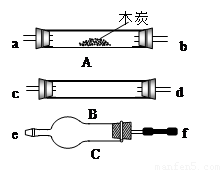
 Cu+H2O��CuO+CO
Cu+H2O��CuO+CO Cu+CO2��
Cu+CO2�� Cu+H2O��CuO+CO
Cu+H2O��CuO+CO Cu+CO2��
Cu+CO2��

 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

 [ʵ�����]������ȷ��
[ʵ�����]������ȷ��| ʵ�鲽�� | ʵ������ | ʵ����� | |
| �٣�19�� | �ڼ��쵼�ܴ���ȼ���壬 �ڼ��쵼�ܴ���ȼ���壬�ڻ����Ϸ���һֻ������ձ� �ڼ��쵼�ܴ���ȼ���壬�ڻ����Ϸ���һֻ������ձ� �� |
�ձ��ڱ���ˮ�� �ձ��ڱ���ˮ�� |
ԭ��������������� ԭ��������������� �� |
| �ڣ�20�� | �������ձ���Ѹ�ٵ�����������ʯ��ˮ�������ڻ����Ϸ���һֻ�ڱ�Ϳ�г���ʯ��ˮ���ձ����� �������ձ���Ѹ�ٵ�����������ʯ��ˮ�������ڻ����Ϸ���һֻ�ڱ�Ϳ�г���ʯ��ˮ���ձ����� �� |
����ʯ��ˮ����ǣ���������ɫ���� ����ʯ��ˮ����ǣ���������ɫ���� |
ԭ�����������һ����̼ ԭ�����������һ����̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ����������б�ҵ��ѧģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
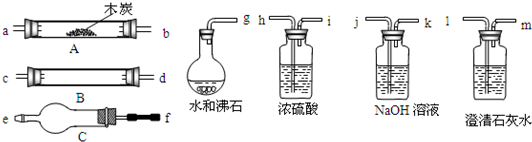
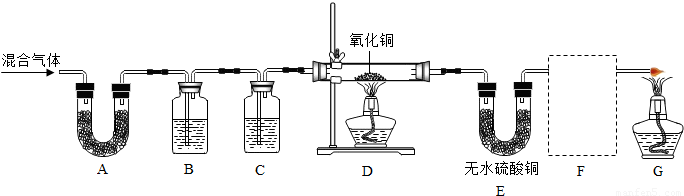
ˮ����ͨ�����ȵ�ľ̿�������������Ҫ�ɷ���CO ��H2������CO2��ˮ�����ȡ�������ͼ���ṩ��������ѡ���Ҫ���Լ������һ��ʵ�飬֤��������������к���CO��H2��������ÿ����Ӧ����ȫ������װ�ú͵��ܵ���ͼ����ȥ��

�ش��������⣺
��1��ʢŨH2SO4��װ����;�� ��ʢNaOH��Һ��װ����;��___��
��2������ B��������Լ������ƣ���ѧʽ���ǣ� ��
��������Ӧ�Ļ�ѧ����ʽ�Ǣ� ��
�� ��
��3���������� C ��������Լ������ƣ���ѧʽ���ǣ� ��
��Ŀ���� _____________ ��
��4���������������Ӹ�����������ĸ��ʾ�ӿڵ�����˳��
g��ab�� ��
��5����֤��������к���CO ��ʵ�������� ��
��6����֤��������к���H2��ʵ��������___________________ ��
�鿴�𰸺ͽ���>>
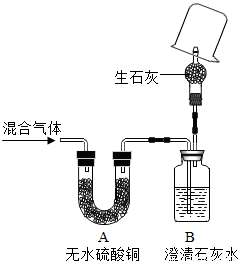
��Ŀ�����л�ѧ ��Դ��2012���Ϻ��г������п���ѧһģ�Ծ��������棩 ���ͣ������
| ʵ�鲽�� | ʵ������ | ʵ����� | |
| �٣�19�� | �ڼ��쵼�ܴ���ȼ���壬______�� | ______ | ______�� |
| �ڣ�20�� | ______�� | ______ | ______ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com